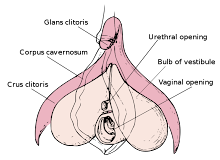
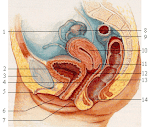
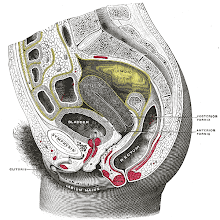
The corpus cavernosum penis is one of a pair of sponge-like regions of erectile tissue which contain most of the blood in the penis during penile erection. This is homologous to the corpus cavernosum clitoridis in the female; the body of the penis contains erectile tissue in a pair of corpora cavernosa (literally "cave-like
bodies"), with a recognisably similar
structure. Anatomy The two corpora cavernosa and corpus spongiosum (also known as the corpus cavernosum urethrae in older texts and in
the diagram to the right) are three
expandable erectile tissues along the
length of the penis which fill with blood during penile erection. The two corpora cavernosa lie along the penis shaft, from
the pubic bones to the head of the penis,
where they join. These formations are
made of a sponge-like tissue containing
irregular blood-filled spaces lined by endothelium and separated by connective tissue septa. [1][2] The male anatomy has no vestibular bulbs, but instead a corpus spongiosum, a smaller region along the bottom of the penis,
which contains the urethra and forms the glans penis. Physiology In some circumstances, release of nitric oxide precedes relaxation of muscles in the corpora cavernosa and corpus spongiosum,
in a process similar to female arousal. The
spongy tissue fills with blood, from arteries
down the length of the penis. A little blood
enters the corpus spongiosum; the
remainder engorges the corpora cavernosa, which expand to hold 90% of the blood
involved in an erection, increasing both in
length and in diameter. The function of the
corpus spongiosum is to prevent
compression of the urethra during erection. Blood can leave the erectile tissue only
through a drainage system of veins around
the outside wall of the corpus cavernosum.
The expanding spongy tissue presses
against a surrounding dense tissue (tunica albuginea) constricting these veins, preventing blood from leaving. The penis
becomes rigid as a result. The glans penis, the expanded cap of the corpus
spongiosum, remains more malleable
during erection because its tunica
albuginea is much thinner than elsewhere
in the penis.
Monday 10 October 2011
CORPUS SPONGIOSUM
Corpus spongiosum (Plural: Corpora Spongiosa) (also known as corpus cavernosum urethrae in older texts) is the mass of spongy tissue surrounding the male urethra within the penis. Although called corpus cavernosum in older texts this
is not correct. Anatomy Behind, it is expanded to form the urethral bulb, and lies in apposition with the inferior fascia of the urogenital diaphragm, from which it receives a fibrous
investment. The urethra enters the bulb nearer to the
superior than to the inferior surface. On the
latter there is a median sulcus (groove),
from which a thin fibrous septum (wall)
projects into the substance of the bulb and
divides it imperfectly into two lateral lobes or hemispheres. The portion of the corpus spongiosum in
front of the bulb lies in a groove on the
under surface of the conjoined corpora
cavernosa penis. It is cylindrical in form
and tapers slightly from behind forward.
Its anterior end is expanded in the form of an obtuse cone, flattened from above
downward. This expansion, termed the glans penis, is moulded on the rounded ends of the corpora cavernosa penis, extending farther on their upper than on
their lower surfaces. At the summit of the glans is the slit-like
vertical external urethral orifice, also known as the meatus. The circumference of the base of the glans
forms a rounded projecting border, the corona glandis, overhanging a deep retroglandular sulcus (Meiring's sulcus),
behind which is the neck of the penis. Function The function of the corpus spongiosum in
erection is to prevent the urethra from
pinching closed, thereby maintaining the
urethra as a viable channel for ejaculation.
To do this, the corpus spongiosum remains
pliable during erection while the corpora cavernosum penis becomes engorged with
blood.
is not correct. Anatomy Behind, it is expanded to form the urethral bulb, and lies in apposition with the inferior fascia of the urogenital diaphragm, from which it receives a fibrous
investment. The urethra enters the bulb nearer to the
superior than to the inferior surface. On the
latter there is a median sulcus (groove),
from which a thin fibrous septum (wall)
projects into the substance of the bulb and
divides it imperfectly into two lateral lobes or hemispheres. The portion of the corpus spongiosum in
front of the bulb lies in a groove on the
under surface of the conjoined corpora
cavernosa penis. It is cylindrical in form
and tapers slightly from behind forward.
Its anterior end is expanded in the form of an obtuse cone, flattened from above
downward. This expansion, termed the glans penis, is moulded on the rounded ends of the corpora cavernosa penis, extending farther on their upper than on
their lower surfaces. At the summit of the glans is the slit-like
vertical external urethral orifice, also known as the meatus. The circumference of the base of the glans
forms a rounded projecting border, the corona glandis, overhanging a deep retroglandular sulcus (Meiring's sulcus),
behind which is the neck of the penis. Function The function of the corpus spongiosum in
erection is to prevent the urethra from
pinching closed, thereby maintaining the
urethra as a viable channel for ejaculation.
To do this, the corpus spongiosum remains
pliable during erection while the corpora cavernosum penis becomes engorged with
blood.
GLANS
The glans penis (or simply glans) is the sensitive bulbous structure at the distal end of the penis. The glans penis is anatomically homologous to the clitoral glans of the female. It is sometimes fully or partially covered by the foreskin, except in men who have been fully circumcised. The glans is also commonly referred to as
the "head of the penis", while common British slang terms include "helmet," "knob end" and "bell end", all referring to its
distinctive shape. The medical name comes
from Latin glans "acorn" + penis "of the penis" – the Latin genitive of this word has the same form as the nominative. Medical considerations The meatus (opening) of the urethra is at the tip of the glans penis. In circumcised infants, the foreskin no longer protects the
meatal area of the glans; consequently,
when wearing diapers, there may be greater risk of developing meatitis, meatal ulceration, and meatal stenosis.[1] The epithelium of the glans penis is mucocutaneous tissue.[2] Birley et al. report that excessive washing with soap
may dry the mucous membrane that covers
the glans penis and cause non-specific dermatitis.[3] Inflammation of the glans penis is known
as balanitis. It occurs in 3–11% of males, and up to 35% of diabetic males. It is more common among uncircumcised males.[4] It has many causes, including irritation, or
infection with a wide variety of
pathogens. Careful identification of the
cause with the aid of patient history,
physical examination, swabs and cultures,
and biopsy are essential in order to determine the proper treatment.[4] Anatomical details The glans penis is the expanded cap of the corpus spongiosum. It is moulded on the rounded ends of the Corpora cavernosa penis, extending farther on their upper than on their lower surfaces. At the
summit of the glans is the slit-like vertical
external urethral orifice. The circumference
of the base of the glans forms a rounded
projecting border, the corona glandis, overhanging a deep retroglandular sulcus (the coronal sulcus), behind which is the
neck of the penis. The proportional size of
the glans penis can vary greatly. On some
penises it is much wider in circumference
than the shaft, giving the penis a mushroom-like appearance, and on others it is narrower and more akin to a probe in
shape. It has been suggested that the
unique and unusual shape of the glans in
humans has evolved to serve the function
of "scooping" any remnant semen deposited by other rival males out of the
deeper part of the vagina of a female who may have recently copulated, and thereby
decreasing the chance of the rival male from impregnating the female.[5] Other theorists[who?] suggest that its distinctive shape evolved to heighten the sexual
pleasure experienced by the female during
vaginal intercourse. In this theory, the
glans increases friction and tension at the
mouth of the vagina by its additional girth and the dilating properties of its probe-like
shape. The foreskin maintains the mucosa in a moist environment.[6] In males who have been circumcised, the glans is permanently exposed and dry. Szabo and Short found
that the glans of the circumcised penis
does not develop a thicker keratinization layer.[7] Several studies have suggested that the glans is equally sensitive in circumcised and uncircumcised males,[8][9] [10][11] while others have reported that it is more sensitive in uncircumcised males [12][13] (the interpretation of one of these studies is disputed[14]). Halata & Munger (1986) report that the
density of genital corpuscles is greatest in the corona glandis,[15] while Yang & Bradley (1998) report that their study
"showed no areas in the glans to be more densely innervated than others." [13] Halata & Spathe (1997) reported that "the
glans penis contains a predominance of
free nerve endings, numerous genital end bulbs and rarely Pacinian and Ruffinian corpuscles. Merkel nerve endings and Meissner's corpuscles are not present."[2] Yang & Bradley argue that "The distinct
pattern of innervation of the glans
emphasizes the role of the glans as a sensory structure".
the "head of the penis", while common British slang terms include "helmet," "knob end" and "bell end", all referring to its
distinctive shape. The medical name comes
from Latin glans "acorn" + penis "of the penis" – the Latin genitive of this word has the same form as the nominative. Medical considerations The meatus (opening) of the urethra is at the tip of the glans penis. In circumcised infants, the foreskin no longer protects the
meatal area of the glans; consequently,
when wearing diapers, there may be greater risk of developing meatitis, meatal ulceration, and meatal stenosis.[1] The epithelium of the glans penis is mucocutaneous tissue.[2] Birley et al. report that excessive washing with soap
may dry the mucous membrane that covers
the glans penis and cause non-specific dermatitis.[3] Inflammation of the glans penis is known
as balanitis. It occurs in 3–11% of males, and up to 35% of diabetic males. It is more common among uncircumcised males.[4] It has many causes, including irritation, or
infection with a wide variety of
pathogens. Careful identification of the
cause with the aid of patient history,
physical examination, swabs and cultures,
and biopsy are essential in order to determine the proper treatment.[4] Anatomical details The glans penis is the expanded cap of the corpus spongiosum. It is moulded on the rounded ends of the Corpora cavernosa penis, extending farther on their upper than on their lower surfaces. At the
summit of the glans is the slit-like vertical
external urethral orifice. The circumference
of the base of the glans forms a rounded
projecting border, the corona glandis, overhanging a deep retroglandular sulcus (the coronal sulcus), behind which is the
neck of the penis. The proportional size of
the glans penis can vary greatly. On some
penises it is much wider in circumference
than the shaft, giving the penis a mushroom-like appearance, and on others it is narrower and more akin to a probe in
shape. It has been suggested that the
unique and unusual shape of the glans in
humans has evolved to serve the function
of "scooping" any remnant semen deposited by other rival males out of the
deeper part of the vagina of a female who may have recently copulated, and thereby
decreasing the chance of the rival male from impregnating the female.[5] Other theorists[who?] suggest that its distinctive shape evolved to heighten the sexual
pleasure experienced by the female during
vaginal intercourse. In this theory, the
glans increases friction and tension at the
mouth of the vagina by its additional girth and the dilating properties of its probe-like
shape. The foreskin maintains the mucosa in a moist environment.[6] In males who have been circumcised, the glans is permanently exposed and dry. Szabo and Short found
that the glans of the circumcised penis
does not develop a thicker keratinization layer.[7] Several studies have suggested that the glans is equally sensitive in circumcised and uncircumcised males,[8][9] [10][11] while others have reported that it is more sensitive in uncircumcised males [12][13] (the interpretation of one of these studies is disputed[14]). Halata & Munger (1986) report that the
density of genital corpuscles is greatest in the corona glandis,[15] while Yang & Bradley (1998) report that their study
"showed no areas in the glans to be more densely innervated than others." [13] Halata & Spathe (1997) reported that "the
glans penis contains a predominance of
free nerve endings, numerous genital end bulbs and rarely Pacinian and Ruffinian corpuscles. Merkel nerve endings and Meissner's corpuscles are not present."[2] Yang & Bradley argue that "The distinct
pattern of innervation of the glans
emphasizes the role of the glans as a sensory structure".
PENIS
The word "penis" is taken from the Latin word for "tail." Some derive that from Indo-European *pesnis, and the Greek word πέος = "penis" from Indo-European
*pesos. Prior to the adoption of the Latin
word in English the penis was referred to
as a "yard". The Oxford English Dictionary cites an example of the word yard used in this sense from 1379,[1] and notes that in his Physical Dictionary of 1684, Steven Blankaart defined the word penis as "the Yard, made up of two nervous Bodies, the Channel, Nut, Skin, and Fore-skin, etc."[2] As with nearly any aspect of the body
involved in sexual or excretory functions, the penis is the subject of taboos, and there are many slang words and euphemisms for it, a particularly common and longstanding one being "cock". The Latin word "phallus" (from Greek φαλλος) is sometimes used to describe the
penis, although "phallus" originally was
used to describe images, pictorial or carved, of the penis.[3] Pizzle, an archaic English word for penis, of Low German or Dutch origin, it is now used
to denote the penis of a non human animal. The adjectival form of the word penis is penile. This adjective is commonly used in describing various accessory structures of
male copulatory organs found in many
kinds of invertebrate animals. In different animals Vertebrates Mammals As with any other bodily attribute, the
length and girth of the penis can be highly
variable between individuals of the same
species. In many animals, especially mammals, the size of a flaccid penis is smaller than its erect size. A bone called the baculum or os penis is present in most mammals but absent in
humans and horses. Domesticated mammals In domestic animals the penis is divided into three parts:[4] Roots (crura): these begin at the caudal border of the pelvic ischial arc. Body: the part of the penis extending
from the roots. Glans: the free end of the penis. The internal structures of the penis consist
mainly of cavernous (erectile) tissue,
which is a collection of blood sinusoids
separated by sheets of connective tissue
(trabeculae). Some animals have a lot of
erectile tissue relative to connective tissue, for example horses. Because of this a
horse's penis can enlarge more than a bull's
penis. The urethra is on the ventral side of the body of the penis. Stallions have a vascular penis. When non-
erect, it is quite flaccid and contained
within the prepuce (sheath). The retractor
penis muscle is relatively underdeveloped.
Erection and protrusion take place
gradually, by the increasing tumescence of the erectile vascular tissue in the corpus cavernosum penis.[5] A bull has a fibro-elastic penis. There is a
small amount of erectile tissue and a small
amount of enlargement after erection. The
penis is quite rigid when non-erect, and
becomes even more rigid during erection.
Protrusion is not affected much by erection, but more by relaxation of the
retractor penis muscle and straightening out of the sigmoid flexure.[5] Dogs have a bulbus glandis at the base of
their penis. During coitus the bulbus glandis
swells up and results in a 'tie' (the male
and female dogs being tied together).
Muscles in the vagina of the female assist
the retention by contracting. The bull, ram and boar have a sigmoid
flexure of their penis. This results in an S-
shaped penis. It is straightened out during
erection. Other mammals As a general rule, an animal's penis is proportional to its body size, but this varies
greatly between species – even between closely related species. For example, an
adult gorilla's erect penis is about 4 cm (1.5 in) in length; an adult chimpanzee, significantly smaller (in body size) than a
gorilla, has a penis size about double that
of the gorilla. In comparison, the human penis is larger than that of any other primate, both in proportion to body size and in absolute terms.[6] In the realm of absolute size, the smallest
vertebrate penis belongs to the Common Shrew (5 mm or 0.2 inches). Accurate measurements of the blue whale are difficult to take because the whale's erect
length can only be observed during mating. [7] Most marsupials, except for the two largest species of kangaroos, have a bifurcated penis. That is, it separates into two
columns, and so the penis has two ends
corresponding to the females' two vaginas. [8] Neither marsupials nor monotremes possess a baculum. Echidnas have a four-headed penis, but only two of the heads are used during
mating. The other two heads "shut down"
and do not grow in size. The heads used
are swapped each time the mammal has sex.[9] Other vertebrates Most male birds (e.g., roosters and turkeys) have a cloaca (also present on the female), but not a penis. Among bird species with a
penis are paleognathes (tinamous and ratites), Anatidae (ducks, geese and swans), and a very few other species (such
as flamingoes). A bird penis is different in structure from mammal penises, being an
erectile expansion of the cloacal wall and
being erected by lymph, not blood. It is usually partially feathered and in some
species features spines and brush-like
filaments, and in flaccid state curls up
inside the cloaca. The Argentine Blue-bill has the largest penis in relation to body
size of all vertebrates; while usually about
half the body size (20 cm), a specimen
with a penis 42.5 cm long is documented. Male specimens of the reptile order Squamata have two paired organs called hemipenes. In some fishes, the gonopodium, andropodium, and claspers are intromittent organs (to introduce sperm into the
female) developed from modified fins. The spine covered penis of Callosobruchus analis, a Bean weevil . Invertebrates The record for the largest penis to body
size ratio is held by the barnacle. The barnacle's penis can grow to up to forty
times its own body length. This enables them to reach the nearest female.[7] In male insects, the structure analogous to a penis is known as aedeagus. The male copulatory organ of various lower
invertebrate animals is often called the
cirrus. A number of invertebrate species have
independently evolved the mating
technique of traumatic insemination where the penis penetrates the female's
abdomen and deposits sperm in the wound it produces. This has been most fully
studied in bedbugs. Cultural uses Culinary, particularly in Chinese
gastronomy (such as dishes from the Guo Li Zhuang Restaurant) Magical and therapeutic, in medicine
and/or superstition, especially as an
alleged aphrodisiac or supposed cure for
impotence – for example the deer penis and tiger penis. Punitive implements, such as the bull pizzle made into a form of whip. Dog chew toys , such as the bull pizzle (cut into short lengths for this purpose).
*pesos. Prior to the adoption of the Latin
word in English the penis was referred to
as a "yard". The Oxford English Dictionary cites an example of the word yard used in this sense from 1379,[1] and notes that in his Physical Dictionary of 1684, Steven Blankaart defined the word penis as "the Yard, made up of two nervous Bodies, the Channel, Nut, Skin, and Fore-skin, etc."[2] As with nearly any aspect of the body
involved in sexual or excretory functions, the penis is the subject of taboos, and there are many slang words and euphemisms for it, a particularly common and longstanding one being "cock". The Latin word "phallus" (from Greek φαλλος) is sometimes used to describe the
penis, although "phallus" originally was
used to describe images, pictorial or carved, of the penis.[3] Pizzle, an archaic English word for penis, of Low German or Dutch origin, it is now used
to denote the penis of a non human animal. The adjectival form of the word penis is penile. This adjective is commonly used in describing various accessory structures of
male copulatory organs found in many
kinds of invertebrate animals. In different animals Vertebrates Mammals As with any other bodily attribute, the
length and girth of the penis can be highly
variable between individuals of the same
species. In many animals, especially mammals, the size of a flaccid penis is smaller than its erect size. A bone called the baculum or os penis is present in most mammals but absent in
humans and horses. Domesticated mammals In domestic animals the penis is divided into three parts:[4] Roots (crura): these begin at the caudal border of the pelvic ischial arc. Body: the part of the penis extending
from the roots. Glans: the free end of the penis. The internal structures of the penis consist
mainly of cavernous (erectile) tissue,
which is a collection of blood sinusoids
separated by sheets of connective tissue
(trabeculae). Some animals have a lot of
erectile tissue relative to connective tissue, for example horses. Because of this a
horse's penis can enlarge more than a bull's
penis. The urethra is on the ventral side of the body of the penis. Stallions have a vascular penis. When non-
erect, it is quite flaccid and contained
within the prepuce (sheath). The retractor
penis muscle is relatively underdeveloped.
Erection and protrusion take place
gradually, by the increasing tumescence of the erectile vascular tissue in the corpus cavernosum penis.[5] A bull has a fibro-elastic penis. There is a
small amount of erectile tissue and a small
amount of enlargement after erection. The
penis is quite rigid when non-erect, and
becomes even more rigid during erection.
Protrusion is not affected much by erection, but more by relaxation of the
retractor penis muscle and straightening out of the sigmoid flexure.[5] Dogs have a bulbus glandis at the base of
their penis. During coitus the bulbus glandis
swells up and results in a 'tie' (the male
and female dogs being tied together).
Muscles in the vagina of the female assist
the retention by contracting. The bull, ram and boar have a sigmoid
flexure of their penis. This results in an S-
shaped penis. It is straightened out during
erection. Other mammals As a general rule, an animal's penis is proportional to its body size, but this varies
greatly between species – even between closely related species. For example, an
adult gorilla's erect penis is about 4 cm (1.5 in) in length; an adult chimpanzee, significantly smaller (in body size) than a
gorilla, has a penis size about double that
of the gorilla. In comparison, the human penis is larger than that of any other primate, both in proportion to body size and in absolute terms.[6] In the realm of absolute size, the smallest
vertebrate penis belongs to the Common Shrew (5 mm or 0.2 inches). Accurate measurements of the blue whale are difficult to take because the whale's erect
length can only be observed during mating. [7] Most marsupials, except for the two largest species of kangaroos, have a bifurcated penis. That is, it separates into two
columns, and so the penis has two ends
corresponding to the females' two vaginas. [8] Neither marsupials nor monotremes possess a baculum. Echidnas have a four-headed penis, but only two of the heads are used during
mating. The other two heads "shut down"
and do not grow in size. The heads used
are swapped each time the mammal has sex.[9] Other vertebrates Most male birds (e.g., roosters and turkeys) have a cloaca (also present on the female), but not a penis. Among bird species with a
penis are paleognathes (tinamous and ratites), Anatidae (ducks, geese and swans), and a very few other species (such
as flamingoes). A bird penis is different in structure from mammal penises, being an
erectile expansion of the cloacal wall and
being erected by lymph, not blood. It is usually partially feathered and in some
species features spines and brush-like
filaments, and in flaccid state curls up
inside the cloaca. The Argentine Blue-bill has the largest penis in relation to body
size of all vertebrates; while usually about
half the body size (20 cm), a specimen
with a penis 42.5 cm long is documented. Male specimens of the reptile order Squamata have two paired organs called hemipenes. In some fishes, the gonopodium, andropodium, and claspers are intromittent organs (to introduce sperm into the
female) developed from modified fins. The spine covered penis of Callosobruchus analis, a Bean weevil . Invertebrates The record for the largest penis to body
size ratio is held by the barnacle. The barnacle's penis can grow to up to forty
times its own body length. This enables them to reach the nearest female.[7] In male insects, the structure analogous to a penis is known as aedeagus. The male copulatory organ of various lower
invertebrate animals is often called the
cirrus. A number of invertebrate species have
independently evolved the mating
technique of traumatic insemination where the penis penetrates the female's
abdomen and deposits sperm in the wound it produces. This has been most fully
studied in bedbugs. Cultural uses Culinary, particularly in Chinese
gastronomy (such as dishes from the Guo Li Zhuang Restaurant) Magical and therapeutic, in medicine
and/or superstition, especially as an
alleged aphrodisiac or supposed cure for
impotence – for example the deer penis and tiger penis. Punitive implements, such as the bull pizzle made into a form of whip. Dog chew toys , such as the bull pizzle (cut into short lengths for this purpose).
INTERSTITIAL CELLS
Interstitial cell refers to any one of a number of different types of cells characterized by their interstitial nature (i.e., their interposition between other
cells that were usually characterized
earlier or more completely.) Examples include: Interstitial cell of Cajal (ICC) Leydig cells, cells present in the male testes responsible for the production of
androgen (male sex hormone) A portion of the stroma of ovary Certain cells in the pineal gland Renal interstitial cells
cells that were usually characterized
earlier or more completely.) Examples include: Interstitial cell of Cajal (ICC) Leydig cells, cells present in the male testes responsible for the production of
androgen (male sex hormone) A portion of the stroma of ovary Certain cells in the pineal gland Renal interstitial cells
SEMINIFEROUS TUBULE
Seminiferous tubules are located in the testes, and are the specific location of meiosis, and the subsequent creation of gametes, namely spermatozoa. The epithelium of the tubule consists of sustentacular or Sertoli cells, which are tall, columnar type cells that line the tubule. In between the Sertoli cells are spermatogenic cells, which differentiate through meiosis to sperm cells. There are two types: convoluted and
straight, convoluted toward the lateral
side, and straight as the tubule comes
medially to form ducts that will exit the
testis. The seminiferous tubules are formed from
primitive sex cords. It is the medullary cords which develop into the seminiferous
tubules and the cortical cords regress. The
cords were formed from the gonadal ridge.
straight, convoluted toward the lateral
side, and straight as the tubule comes
medially to form ducts that will exit the
testis. The seminiferous tubules are formed from
primitive sex cords. It is the medullary cords which develop into the seminiferous
tubules and the cortical cords regress. The
cords were formed from the gonadal ridge.
SUSTENTACULAR CELL
A sustentacular cell is a type of cell primarily associated with structural
support. One type of sustentacular cell is the Sertoli cell, in the testicle. It is located in the walls of the seminiferous tubules and supplies
nutrients to sperm. Another type of sustentacular cell is found
in the Olfactory epithelium.The Internal Ear (Organ of Corti) and the taste buds also
contain the sustentacular cell. About 40% of carcinoids have a scattering
of sustentacular cells, which stain positive
for S-100.
support. One type of sustentacular cell is the Sertoli cell, in the testicle. It is located in the walls of the seminiferous tubules and supplies
nutrients to sperm. Another type of sustentacular cell is found
in the Olfactory epithelium.The Internal Ear (Organ of Corti) and the taste buds also
contain the sustentacular cell. About 40% of carcinoids have a scattering
of sustentacular cells, which stain positive
for S-100.
SPERM CELLS
The term sperm is derived from the Greek word (σπέρμα) sperma (meaning "seed")
and refers to the male reproductive cells. In the types of sexual reproduction known as anisogamy and oogamy, there is a marked difference in the size of the gametes with the smaller one being termed the "male" or
sperm cell. The human sperm cell is haploid, so that its 23 chromosomes can join the 23
chromosomes of the female egg to form a diploid cell. A uniflagellar sperm cell that is motile is referred to as a spermatozoon, whereas a non-motile sperm cell is
referred to as a spermatium. Sperm cells cannot divide and have a limited life span,
but after fusion with egg cells during fertilization, a new organism begins
developing, starting as a totipotent zygote.[citation needed] The spermatozoa of animals are produced through spermatogenesis inside the male gonads (testicles) via meiotic division. They are carried out of the male body in a
fluid known as semen. Mammalian sperm cells can survive within the female
reproductive tract for more than 5 days post coitus.[1] Sperm cells in algal and many plant gametophytes are produced in male gametangia (antheridia) via mitotic division. In flowering plants, sperm nuclei are produced inside pollen.[citation needed] Etymology The term "sperm" probably comes from
sperma which in Greek is "seed" or Latin
"something sown". Other terms for sperm
include "prostatic fluid" and "seminal fluid"
and "seed". Origin Sperm originates solely from the testicles, and this is where sperm develop. The
initial spermatozoon process takes around 70 days to complete. The spermatid stage is where the sperm develops the familiar
tail. The next stage where it becomes fully
mature takes around 60 days when its called a spermatozoan.[2] Subsequently, the semen wherein the sperm is carried is produced in the seminal vesicles, prostate gland and urethral glands. AnatomySperm fertilizing an egg The sperm cell consists of a head, a
midpiece and a tail. The head contains the nucleus with densely coiled chromatin fibres, surrounded anteriorly by an acrosome, which contains enzymes used for penetrating the female egg. The
midpiece has a central filamentous core
with many mitochondria spiralled around
it, used for ATP production for the journey through the female cervix, uterus and uterine tubes. The tail or "flagellum" executes the lashing movements that propel the spermatocyte.[citation needed] During fertilization, the sperm provides three essential parts to the oocyte: (1) a signalling or activating factor, which
causes the metabolically dormant oocyte to activate; (2) the haploid paternal genome; (3) the centrosome, which is responsible for maintaining the microtubule system.[3] Motile sperm cells Motile sperm cells of algae and seedless plants.[4] Motile sperm cells typically move via flagella and require a water medium in order to swim toward the egg for
fertilization. Most of the energy for sperm
motility is derived from the metabolism of fructose carried in the seminal fluid. This takes place in the mitochondria located in the sperm's midpiece (at the base of the
sperm head). These cells cannot swim
backwards due to the nature of their
propulsion. The uniflagellated sperm cells
(with one flagellum) produced in most animals are referred to as spermatozoa, and are known to vary in size.[citation needed] Motile sperm are also produced by many protists and the gametophytes of bryophytes , ferns and some gymnosperms such as cycads and ginkgo. The sperm cells are the only flagellated cells in the life
cycle of these plants. In many ferns and lycophytes , they are multi-flagellated (carrying more than one flagellum).[4] In nematodes, the sperm cells are amoeboid and crawl, rather than swim, towards the egg cell.[5] Non-motile sperm cells Non-motile sperm cells called spermatia lack flagella and therefore cannot swim.
Spermatia are produced in a spermatangium.[4] Because spermatia cannot swim, they
depend on their environment to carry them
to the egg cell. Some red algae, such as Polysiphonia, produce non-motile spermatia that are spread by water currents after their release.[4] The spermatia of rust fungi are covered with a sticky substance. They are produced in
flask-shaped structures containing nectar, which attract flies that transfer the spermatia to nearby hyphae for fertilization in a mechanism similar to insect pollination in flowering plants.[6] Fungal spermatia (also called pycniospores,
especially in the Uredinales) may be
confused with conidia. Conidia are spores that germinate independently of
fertilization, whereas spermatia are gametes that are required for fertilization. In some fungi, such as Neurospora crassa, spermatia are identical to microconidia as
they can perform both functions of
fertilization as well as giving rise to new organisms without fertilization.[7] Sperm nuclei In many land plants, including most gymnosperms and all angiosperms, the male gametophytes (pollen grains) are the primary mode of dispersal, for example via wind or insect pollination, eliminating the need for water to bridge the gap between
male and female. Each pollen grain
contains a spermatogenous (generative)
cell. Once the pollen lands on the stigma of a receptive flower, it germinates and
starts growing a pollen tube through the carpel. Before the tube reaches the ovule, the nucleus of the generative cell in the
pollen grain divides and gives rise to two
sperm nuclei which are then discharged
through the tube into the ovule for fertilization.[4] In some protists, fertilization also involves sperm nuclei, rather than cells, migrating toward the egg cell through a fertilization
tube. Oomycetes form sperm nuclei in a syncytical antheridium surrounding the egg cells. The sperm nuclei reach the eggs
through fertilization tubes, similar to the pollen tube mechanism in plants.[4] Sperm quality Human sperm stained for semen quality testing. Main article: Semen quality Sperm quantity and quality are the main parameters in semen quality, which is a measure of the ability of semen to accomplish fertilization. Thus, in humans, it is a measure of fertility in a man. The genetic quality of sperm, as well as its
volume and motility, all typically decrease with age.[8] (See paternal age effect.) Market for human sperm Further information: Sperm donation On the global market, Denmark has a well- developed system of human sperm export.
This success mainly comes from the
reputation of Danish sperm donors for being of high quality[9] and, in contrast with the law in the other Nordic countries,
gives donors the choice of being either
anonymous or non-anonymous to the receiving couple.[9] Furthermore, Nordic sperm donors tend to be tall and highly educated[10] and have altruistic motives for their donations,[10] partly due to the relatively low monetary compensation in
Nordic countries. More than 50 countries
worldwide are importers of Danish sperm,
including Paraguay, Canada, Kenya, and Hong Kong.[9] However, the Food and Drug Administration (FDA) of the US has banned import of any sperm, motivated by a risk
of mad cow disease, although such a risk is insignificant, since artificial insemination
is very different from the route of transmission of mad cow disease.[11] The prevalence of mad cow disease is one in a million, probably less for donors. If
prevalence was the case, the infectious
proteins would then have to cross the blood-testis barrier to make transmission possible.[11] Transmission of the disease by an insemination is approximately equal
to the risk of getting killed by lightning. [12] History See also: Homunculus#Homunculus of spermists Sperm were first observed in 1677 by Antonie van Leeuwenhoek [13] using a microscope, he described them as being animalcules (little animals), probably due to his belief in preformationism, which thought that each sperm contained a fully formed but small human.[citation needed] Forensic Analysis Ejaculated fluids are detected by ultraviolet light, irrespective of the structure or colour of the surface.[14] Sperm heads, e.g. from vaginal swabs, are
still detected by microscopy using the "Christmas Tree Stain" method, i.e.,
Kernechtrot-Picroindigocarmine (KPIC) staining
and refers to the male reproductive cells. In the types of sexual reproduction known as anisogamy and oogamy, there is a marked difference in the size of the gametes with the smaller one being termed the "male" or
sperm cell. The human sperm cell is haploid, so that its 23 chromosomes can join the 23
chromosomes of the female egg to form a diploid cell. A uniflagellar sperm cell that is motile is referred to as a spermatozoon, whereas a non-motile sperm cell is
referred to as a spermatium. Sperm cells cannot divide and have a limited life span,
but after fusion with egg cells during fertilization, a new organism begins
developing, starting as a totipotent zygote.[citation needed] The spermatozoa of animals are produced through spermatogenesis inside the male gonads (testicles) via meiotic division. They are carried out of the male body in a
fluid known as semen. Mammalian sperm cells can survive within the female
reproductive tract for more than 5 days post coitus.[1] Sperm cells in algal and many plant gametophytes are produced in male gametangia (antheridia) via mitotic division. In flowering plants, sperm nuclei are produced inside pollen.[citation needed] Etymology The term "sperm" probably comes from
sperma which in Greek is "seed" or Latin
"something sown". Other terms for sperm
include "prostatic fluid" and "seminal fluid"
and "seed". Origin Sperm originates solely from the testicles, and this is where sperm develop. The
initial spermatozoon process takes around 70 days to complete. The spermatid stage is where the sperm develops the familiar
tail. The next stage where it becomes fully
mature takes around 60 days when its called a spermatozoan.[2] Subsequently, the semen wherein the sperm is carried is produced in the seminal vesicles, prostate gland and urethral glands. AnatomySperm fertilizing an egg The sperm cell consists of a head, a
midpiece and a tail. The head contains the nucleus with densely coiled chromatin fibres, surrounded anteriorly by an acrosome, which contains enzymes used for penetrating the female egg. The
midpiece has a central filamentous core
with many mitochondria spiralled around
it, used for ATP production for the journey through the female cervix, uterus and uterine tubes. The tail or "flagellum" executes the lashing movements that propel the spermatocyte.[citation needed] During fertilization, the sperm provides three essential parts to the oocyte: (1) a signalling or activating factor, which
causes the metabolically dormant oocyte to activate; (2) the haploid paternal genome; (3) the centrosome, which is responsible for maintaining the microtubule system.[3] Motile sperm cells Motile sperm cells of algae and seedless plants.[4] Motile sperm cells typically move via flagella and require a water medium in order to swim toward the egg for
fertilization. Most of the energy for sperm
motility is derived from the metabolism of fructose carried in the seminal fluid. This takes place in the mitochondria located in the sperm's midpiece (at the base of the
sperm head). These cells cannot swim
backwards due to the nature of their
propulsion. The uniflagellated sperm cells
(with one flagellum) produced in most animals are referred to as spermatozoa, and are known to vary in size.[citation needed] Motile sperm are also produced by many protists and the gametophytes of bryophytes , ferns and some gymnosperms such as cycads and ginkgo. The sperm cells are the only flagellated cells in the life
cycle of these plants. In many ferns and lycophytes , they are multi-flagellated (carrying more than one flagellum).[4] In nematodes, the sperm cells are amoeboid and crawl, rather than swim, towards the egg cell.[5] Non-motile sperm cells Non-motile sperm cells called spermatia lack flagella and therefore cannot swim.
Spermatia are produced in a spermatangium.[4] Because spermatia cannot swim, they
depend on their environment to carry them
to the egg cell. Some red algae, such as Polysiphonia, produce non-motile spermatia that are spread by water currents after their release.[4] The spermatia of rust fungi are covered with a sticky substance. They are produced in
flask-shaped structures containing nectar, which attract flies that transfer the spermatia to nearby hyphae for fertilization in a mechanism similar to insect pollination in flowering plants.[6] Fungal spermatia (also called pycniospores,
especially in the Uredinales) may be
confused with conidia. Conidia are spores that germinate independently of
fertilization, whereas spermatia are gametes that are required for fertilization. In some fungi, such as Neurospora crassa, spermatia are identical to microconidia as
they can perform both functions of
fertilization as well as giving rise to new organisms without fertilization.[7] Sperm nuclei In many land plants, including most gymnosperms and all angiosperms, the male gametophytes (pollen grains) are the primary mode of dispersal, for example via wind or insect pollination, eliminating the need for water to bridge the gap between
male and female. Each pollen grain
contains a spermatogenous (generative)
cell. Once the pollen lands on the stigma of a receptive flower, it germinates and
starts growing a pollen tube through the carpel. Before the tube reaches the ovule, the nucleus of the generative cell in the
pollen grain divides and gives rise to two
sperm nuclei which are then discharged
through the tube into the ovule for fertilization.[4] In some protists, fertilization also involves sperm nuclei, rather than cells, migrating toward the egg cell through a fertilization
tube. Oomycetes form sperm nuclei in a syncytical antheridium surrounding the egg cells. The sperm nuclei reach the eggs
through fertilization tubes, similar to the pollen tube mechanism in plants.[4] Sperm quality Human sperm stained for semen quality testing. Main article: Semen quality Sperm quantity and quality are the main parameters in semen quality, which is a measure of the ability of semen to accomplish fertilization. Thus, in humans, it is a measure of fertility in a man. The genetic quality of sperm, as well as its
volume and motility, all typically decrease with age.[8] (See paternal age effect.) Market for human sperm Further information: Sperm donation On the global market, Denmark has a well- developed system of human sperm export.
This success mainly comes from the
reputation of Danish sperm donors for being of high quality[9] and, in contrast with the law in the other Nordic countries,
gives donors the choice of being either
anonymous or non-anonymous to the receiving couple.[9] Furthermore, Nordic sperm donors tend to be tall and highly educated[10] and have altruistic motives for their donations,[10] partly due to the relatively low monetary compensation in
Nordic countries. More than 50 countries
worldwide are importers of Danish sperm,
including Paraguay, Canada, Kenya, and Hong Kong.[9] However, the Food and Drug Administration (FDA) of the US has banned import of any sperm, motivated by a risk
of mad cow disease, although such a risk is insignificant, since artificial insemination
is very different from the route of transmission of mad cow disease.[11] The prevalence of mad cow disease is one in a million, probably less for donors. If
prevalence was the case, the infectious
proteins would then have to cross the blood-testis barrier to make transmission possible.[11] Transmission of the disease by an insemination is approximately equal
to the risk of getting killed by lightning. [12] History See also: Homunculus#Homunculus of spermists Sperm were first observed in 1677 by Antonie van Leeuwenhoek [13] using a microscope, he described them as being animalcules (little animals), probably due to his belief in preformationism, which thought that each sperm contained a fully formed but small human.[citation needed] Forensic Analysis Ejaculated fluids are detected by ultraviolet light, irrespective of the structure or colour of the surface.[14] Sperm heads, e.g. from vaginal swabs, are
still detected by microscopy using the "Christmas Tree Stain" method, i.e.,
Kernechtrot-Picroindigocarmine (KPIC) staining
spermatids
The spermatid is the haploid male gametid that results from division of secondary spermatocytes. As a result of meiosis, each spermatid contains only half of the genetic
material present in the original primary
spermatocyte. Spermatids are connected together by
cytoplasmic material and have superfluous
cytoplasmic material around their nuclei. When formed, early round spermatids must
undergo further maturational events in
order to develop into spermatozoa, a process termed spermiogenesis (also termed spermeteliosis). The spermatids begin to grow a living
thread, develop a thickened mid-piece
where the mitochondria become localised, and form an acrosome. Spermatid DNA also undergoes packaging, becoming highly
condensed. The DNA is packaged firstly
with specific nuclear basic proteins, which
are subsequently replaced with protamines during spermatid elongation. The resultant
tightly packed chromatin is transcriptionally inactive.
material present in the original primary
spermatocyte. Spermatids are connected together by
cytoplasmic material and have superfluous
cytoplasmic material around their nuclei. When formed, early round spermatids must
undergo further maturational events in
order to develop into spermatozoa, a process termed spermiogenesis (also termed spermeteliosis). The spermatids begin to grow a living
thread, develop a thickened mid-piece
where the mitochondria become localised, and form an acrosome. Spermatid DNA also undergoes packaging, becoming highly
condensed. The DNA is packaged firstly
with specific nuclear basic proteins, which
are subsequently replaced with protamines during spermatid elongation. The resultant
tightly packed chromatin is transcriptionally inactive.
SPERMATOGONIUM
A spermatogonium (plural: spermatogonia) is an intermediary male gametogonium (a kind of germ cell) in the production of spermatozoa. There are three subtypes: Type A(d) cells, with dark nuclei. These cells replicate to ensure a constant
supply of spermatogonia to fuel
spermatogenesis. Type A(p) cells, with pale nuclei. These cells divide by mitosis to produce Type B
cells. Type B cells, which divide to give rise to primary spermatocytes. Each primary spermatocyte duplicates its
DNA and subsequently undergoes meiosis I to produce two haploid secondary
spermatocytes. Each of the two secondary
spermatocytes further undergo meiosis II
to produce two spermatids (haploid). (1
primary spermatocyte => 4 spermatids) The spermatids then undergo spermiogenesis to produce spermatozoa.
supply of spermatogonia to fuel
spermatogenesis. Type A(p) cells, with pale nuclei. These cells divide by mitosis to produce Type B
cells. Type B cells, which divide to give rise to primary spermatocytes. Each primary spermatocyte duplicates its
DNA and subsequently undergoes meiosis I to produce two haploid secondary
spermatocytes. Each of the two secondary
spermatocytes further undergo meiosis II
to produce two spermatids (haploid). (1
primary spermatocyte => 4 spermatids) The spermatids then undergo spermiogenesis to produce spermatozoa.
URINARY SYSTEM
The urinary system (also called the excretory system ) is the organ system that produces, stores, and eliminates urine. In humans it includes two kidneys, two ureters, the bladder and the urethra. Physiology of urinary system Kidney Main article: Kidney The kidneys are bean-shaped organs that
lie in the abdomen, retroperitoneal to the organs of digestion, around or just below
the ribcage and close to the lumbar spine. The organ is about the size of a human fist
and is surrounded by what is called Peri-
nephric fat, and situated on the superior
pole of each kidney is an adrenal gland. The kidneys receive their blood supply of 1.25 L/min (25% of the cardiac output) from the renal arteries which are fed by
the abdominal aorta. This is important because the kidneys' main role is to filter water soluble waste products from the blood. The other attachment of the kidneys
are at their functional endpoints the ureters, which lies more medial and runs down to the trigone of urinary bladder. The kidneys perform a number of tasks, such as: concentrating urine, regulating electrolytes, and maintaining acid-base homeostasis. The kidney excretes and re- absorbs electrolytes (e.g. sodium, potassium and calcium) under the influence of local and systemic hormones. pH balance is regulated by the excretion of bound acids and ammonium ions. In addition, they remove urea, a nitrogenous waste product from the metabolism of amino acids. The end point is a hyperosmolar solution carrying waste for storage in the
bladder prior to urination. Humans produce about 2.9 litres of urine over 24 hours, although this amount may
vary according to circumstances. Because
the rate of filtration at the kidney is proportional to the glomerular filtration rate, which is in turn related to the blood flow through the kidney, changes in body
fluid status can affect kidney function.
Hormones exogenous and endogenous to
the kidney alter the amount of blood flowing through the glomerulus. Some medications interfere directly or indirectly with urine production. Diuretics achieve this by altering the amount of absorbed or
excreted electrolytes or osmalites, which causes a diuresis.
lie in the abdomen, retroperitoneal to the organs of digestion, around or just below
the ribcage and close to the lumbar spine. The organ is about the size of a human fist
and is surrounded by what is called Peri-
nephric fat, and situated on the superior
pole of each kidney is an adrenal gland. The kidneys receive their blood supply of 1.25 L/min (25% of the cardiac output) from the renal arteries which are fed by
the abdominal aorta. This is important because the kidneys' main role is to filter water soluble waste products from the blood. The other attachment of the kidneys
are at their functional endpoints the ureters, which lies more medial and runs down to the trigone of urinary bladder. The kidneys perform a number of tasks, such as: concentrating urine, regulating electrolytes, and maintaining acid-base homeostasis. The kidney excretes and re- absorbs electrolytes (e.g. sodium, potassium and calcium) under the influence of local and systemic hormones. pH balance is regulated by the excretion of bound acids and ammonium ions. In addition, they remove urea, a nitrogenous waste product from the metabolism of amino acids. The end point is a hyperosmolar solution carrying waste for storage in the
bladder prior to urination. Humans produce about 2.9 litres of urine over 24 hours, although this amount may
vary according to circumstances. Because
the rate of filtration at the kidney is proportional to the glomerular filtration rate, which is in turn related to the blood flow through the kidney, changes in body
fluid status can affect kidney function.
Hormones exogenous and endogenous to
the kidney alter the amount of blood flowing through the glomerulus. Some medications interfere directly or indirectly with urine production. Diuretics achieve this by altering the amount of absorbed or
excreted electrolytes or osmalites, which causes a diuresis.
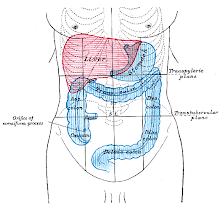
DIAPHRAGM
In the anatomy of mammals, the thoracic diaphragm, or simply the diaphragm (Ancient Greek: διάφραγμα diáphragma "partition"), is a sheet of internal skeletal muscle[2] that extends across the bottom of the rib cage. The diaphragm separates the thoracic cavity (heart, lungs & ribs) from the abdominal cavity and performs an important function in respiration. A diaphragm in anatomy can refer to other flat structures such as the urogenital diaphragm or pelvic diaphragm, but "the diaphragm" generally refers to the thoracic
diaphragm. Other vertebrates such as amphibians and reptiles have diaphragms or diaphragm-like structures, but important
details of the anatomy vary, such as the
position of lungs in the abdominal cavity. Function The diaphragm functions in breathing. During inhalation, the diaphragm contracts,
thus enlarging the thoracic cavity (the external intercostal muscles also participate in this enlargement). This
reduces intra-thoracic pressure: In other
words, enlarging the cavity creates suction
that draws air into the lungs. Cavity expansion happens in two
extremes, along with intermediary forms.
When the lower ribs are stabilized and the
central tendon of the diaphragm is mobile,
a contraction brings the insertion (central
tendon) towards the origins and pushes the lower cavity towards the pelvis,
allowing the thoracic cavity to expand
downward. This is often called belly breathing. When the central tendon is stabilized and the lower ribs are mobile, a
contraction lifts the origins (ribs) up
towards the insertion (central tendon)
which works in conjunction with other
muscles to allow the ribs to slide and the
thoracic cavity to expand laterally and upwards. When the diaphragm relaxes, air is exhaled
by elastic recoil of the lung and the tissues
lining the thoracic cavity. Assisting this
function with muscular effort (called forced exhalation) involves the internal intercostal muscles used in conjunction with the abdominal muscles, which act as an antagonist paired with the diaphragm's contraction. The diaphragm is also involved in non-
respiratory functions, helping to expel vomit, feces, and urine from the body by increasing intra-abdominal pressure, and
preventing acid reflux by exerting pressure on the esophagus as it passes through the esophageal hiatus. In some non-human animals, the
diaphragm is not crucial for breathing; a
cow, for instance, can survive fairly
asymptomatically with diaphragmatic
paralysis as long as no massive aerobic
metabolic demands are made of it. Anatomy The diaphragm is a dome-shaped
musculofibrous septum that separates the
thoracic from the abdominal cavity, its
convex upper surface forming the floor of
the former, and its concave under surface
forming the roof of the latter. Its peripheral part consists of muscular fibers
that take origin from the circumference of
the inferior thoracic aperture and converge to be inserted into a central tendon.
diaphragm. Other vertebrates such as amphibians and reptiles have diaphragms or diaphragm-like structures, but important
details of the anatomy vary, such as the
position of lungs in the abdominal cavity. Function The diaphragm functions in breathing. During inhalation, the diaphragm contracts,
thus enlarging the thoracic cavity (the external intercostal muscles also participate in this enlargement). This
reduces intra-thoracic pressure: In other
words, enlarging the cavity creates suction
that draws air into the lungs. Cavity expansion happens in two
extremes, along with intermediary forms.
When the lower ribs are stabilized and the
central tendon of the diaphragm is mobile,
a contraction brings the insertion (central
tendon) towards the origins and pushes the lower cavity towards the pelvis,
allowing the thoracic cavity to expand
downward. This is often called belly breathing. When the central tendon is stabilized and the lower ribs are mobile, a
contraction lifts the origins (ribs) up
towards the insertion (central tendon)
which works in conjunction with other
muscles to allow the ribs to slide and the
thoracic cavity to expand laterally and upwards. When the diaphragm relaxes, air is exhaled
by elastic recoil of the lung and the tissues
lining the thoracic cavity. Assisting this
function with muscular effort (called forced exhalation) involves the internal intercostal muscles used in conjunction with the abdominal muscles, which act as an antagonist paired with the diaphragm's contraction. The diaphragm is also involved in non-
respiratory functions, helping to expel vomit, feces, and urine from the body by increasing intra-abdominal pressure, and
preventing acid reflux by exerting pressure on the esophagus as it passes through the esophageal hiatus. In some non-human animals, the
diaphragm is not crucial for breathing; a
cow, for instance, can survive fairly
asymptomatically with diaphragmatic
paralysis as long as no massive aerobic
metabolic demands are made of it. Anatomy The diaphragm is a dome-shaped
musculofibrous septum that separates the
thoracic from the abdominal cavity, its
convex upper surface forming the floor of
the former, and its concave under surface
forming the roof of the latter. Its peripheral part consists of muscular fibers
that take origin from the circumference of
the inferior thoracic aperture and converge to be inserted into a central tendon.
ADRENAL GLAND
In mammals, the adrenal glands (also known as suprarenal glands) are endocrine glands that sit atop the kidneys; in humans, the right suprarenal gland is
triangular shaped, while the left suprarenal
gland is semilunar shaped. They are chiefly
responsible for releasing hormones in response to stress through the synthesis of corticosteroids such as cortisol and catecholamines such as epinephrine. The adrenal glands affect kidney function
through the secretion of aldosterone, a hormone involved in regulating the osmolarity of blood plasma. Anatomy and Physiology Anatomically, the adrenal glands are
located in the retroperitoneum situated atop the kidneys, one on each side. They are surrounded by an adipose capsule and renal fascia. In humans, the adrenal glands are found at the level of the 12th thoracic vertebra. Each adrenal gland has two distinct structures, the adrenal cortex and the medulla, both of which produce hormones. The cortex mainly produces cortisol, aldosterone and androgens, while the medulla chiefly produces epinephrine and norepinephrine. The combined weight of the adrenal glands in an adult human ranges from 7 to 10 grams.[1] A CT scan in which the Adrenals are shown as the triangular-shaped organs on top of the kidneys Cortex The adrenal cortex is devoted to the synthesis of corticosteroid hormones. Specific cortical cells produce particular
hormones including cortisol, corticosterone, androgens such as testosterone, and aldosterone. Under normal unstressed conditions, the human adrenal glands
produce the equivalent of 35–40 mg of cortisone acetate per day.[2] In contrast to the direct innervation of the medulla, the
cortex is regulated by neuroendocrine hormones secreted by the pituitary gland and hypothalamus, as well as by the renin- angiotensin system. The adrenal cortex comprises three zones,
or layers. This anatomic zonation can be
appreciated at the microscopic level,
where each zone can be recognized and
distinguished from one another based on structural and anatomic characteristics.[3] The adrenal cortex exhibits functional
zonation as well: by virtue of the
characteristic enzymes present in each
zone, the zones produce and secrete distinct hormones.[3] Zona glomerulosa (outer) The outermost layer, the zona glomerulosa is the main site for production of mineralocorticoids, mainly aldosterone, which is largely responsible for the long-term regulation of blood pressure. Zona fasciculata Situated between the glomerulosa and
reticularis, the zona fasciculata is responsible for producing glucocorticoids, chiefly cortisol in humans. The zona fasciculata secretes a
basal level of cortisol but can also
produce bursts of the hormone in
response to adrenocorticotropic hormone (ACTH) from the anterior pituitary. Zona reticularis The inner most cortical layer, the zona reticularis produces androgens, mainly dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEA-S) in humans. Medulla The adrenal medulla is the core of the adrenal gland, and is surrounded by the
adrenal cortex. The chromaffin cells of the medulla, named for their characteristic
brown staining with chromic acid salts, are the body's main source of the circulating catecholamines adrenaline (epinephrine) and noradrenaline (norepinephrine). Derived from the amino acid tyrosine, these water-soluble hormones are major
hormones underlying the fight-or-flight response. To carry out its part of this response, the
adrenal medulla receives input from the sympathetic nervous system through preganglionic fibers originating in the thoracic spinal cord from T5–T11.[4] Because it is innervated by preganglionic
nerve fibers, the adrenal medulla can be
considered as a specialized sympathetic ganglion.[4] Unlike other sympathetic ganglia, however, the adrenal medulla
lacks distinct synapses and releases its
secretions directly into the blood. Cortisol also promotes epinephrine
synthesis in the medulla. Produced in the
cortex, cortisol reaches the adrenal medulla
and at high levels, the hormone can
promote the upregulation of phenylethanolamine N-methyltransferase (PNMT), thereby increasing epinephrine synthesis and secretion.[3] Blood supply Although variations of the blood supply to
the adrenal glands (and indeed the kidneys
themselves) are common, there are usually
three arteries that supply each adrenal
gland: The superior suprarenal artery is provided by the inferior phrenic artery The middle suprarenal artery is provided by the abdominal aorta The inferior suprarenal artery is provided by the renal artery Venous drainage of the adrenal glands is achieved via the suprarenal veins: The right suprarenal vein drains into the inferior vena cava The left suprarenal vein drains into the left renal vein or the left inferior phrenic vein. The suprarenal veins may form anastomoses with the inferior phrenic veins. Since the right supra-renal vein is short and drains directly into the inferior
vena cava it is likely to injure the latter
during removal of right adrenal for various
reasons. The adrenal glands and the thyroid gland are the organs that have the greatest blood
supply per gram of tissue. Up to 60 arterioles may enter each adrenal gland.[5] This may be one of the reasons lung cancer
commonly metastasizes to the adrenals. Terminology The adrenal glands are named for their
location relative to the kidneys. The term
"adrenal" comes from ad- (Latin, "near")
and renes (Latin, "kidney"). Similarly,
"suprarenal" is derived from supra- (Latin,
"above") and renes.
triangular shaped, while the left suprarenal
gland is semilunar shaped. They are chiefly
responsible for releasing hormones in response to stress through the synthesis of corticosteroids such as cortisol and catecholamines such as epinephrine. The adrenal glands affect kidney function
through the secretion of aldosterone, a hormone involved in regulating the osmolarity of blood plasma. Anatomy and Physiology Anatomically, the adrenal glands are
located in the retroperitoneum situated atop the kidneys, one on each side. They are surrounded by an adipose capsule and renal fascia. In humans, the adrenal glands are found at the level of the 12th thoracic vertebra. Each adrenal gland has two distinct structures, the adrenal cortex and the medulla, both of which produce hormones. The cortex mainly produces cortisol, aldosterone and androgens, while the medulla chiefly produces epinephrine and norepinephrine. The combined weight of the adrenal glands in an adult human ranges from 7 to 10 grams.[1] A CT scan in which the Adrenals are shown as the triangular-shaped organs on top of the kidneys Cortex The adrenal cortex is devoted to the synthesis of corticosteroid hormones. Specific cortical cells produce particular
hormones including cortisol, corticosterone, androgens such as testosterone, and aldosterone. Under normal unstressed conditions, the human adrenal glands
produce the equivalent of 35–40 mg of cortisone acetate per day.[2] In contrast to the direct innervation of the medulla, the
cortex is regulated by neuroendocrine hormones secreted by the pituitary gland and hypothalamus, as well as by the renin- angiotensin system. The adrenal cortex comprises three zones,
or layers. This anatomic zonation can be
appreciated at the microscopic level,
where each zone can be recognized and
distinguished from one another based on structural and anatomic characteristics.[3] The adrenal cortex exhibits functional
zonation as well: by virtue of the
characteristic enzymes present in each
zone, the zones produce and secrete distinct hormones.[3] Zona glomerulosa (outer) The outermost layer, the zona glomerulosa is the main site for production of mineralocorticoids, mainly aldosterone, which is largely responsible for the long-term regulation of blood pressure. Zona fasciculata Situated between the glomerulosa and
reticularis, the zona fasciculata is responsible for producing glucocorticoids, chiefly cortisol in humans. The zona fasciculata secretes a
basal level of cortisol but can also
produce bursts of the hormone in
response to adrenocorticotropic hormone (ACTH) from the anterior pituitary. Zona reticularis The inner most cortical layer, the zona reticularis produces androgens, mainly dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEA-S) in humans. Medulla The adrenal medulla is the core of the adrenal gland, and is surrounded by the
adrenal cortex. The chromaffin cells of the medulla, named for their characteristic
brown staining with chromic acid salts, are the body's main source of the circulating catecholamines adrenaline (epinephrine) and noradrenaline (norepinephrine). Derived from the amino acid tyrosine, these water-soluble hormones are major
hormones underlying the fight-or-flight response. To carry out its part of this response, the
adrenal medulla receives input from the sympathetic nervous system through preganglionic fibers originating in the thoracic spinal cord from T5–T11.[4] Because it is innervated by preganglionic
nerve fibers, the adrenal medulla can be
considered as a specialized sympathetic ganglion.[4] Unlike other sympathetic ganglia, however, the adrenal medulla
lacks distinct synapses and releases its
secretions directly into the blood. Cortisol also promotes epinephrine
synthesis in the medulla. Produced in the
cortex, cortisol reaches the adrenal medulla
and at high levels, the hormone can
promote the upregulation of phenylethanolamine N-methyltransferase (PNMT), thereby increasing epinephrine synthesis and secretion.[3] Blood supply Although variations of the blood supply to
the adrenal glands (and indeed the kidneys
themselves) are common, there are usually
three arteries that supply each adrenal
gland: The superior suprarenal artery is provided by the inferior phrenic artery The middle suprarenal artery is provided by the abdominal aorta The inferior suprarenal artery is provided by the renal artery Venous drainage of the adrenal glands is achieved via the suprarenal veins: The right suprarenal vein drains into the inferior vena cava The left suprarenal vein drains into the left renal vein or the left inferior phrenic vein. The suprarenal veins may form anastomoses with the inferior phrenic veins. Since the right supra-renal vein is short and drains directly into the inferior
vena cava it is likely to injure the latter
during removal of right adrenal for various
reasons. The adrenal glands and the thyroid gland are the organs that have the greatest blood
supply per gram of tissue. Up to 60 arterioles may enter each adrenal gland.[5] This may be one of the reasons lung cancer
commonly metastasizes to the adrenals. Terminology The adrenal glands are named for their
location relative to the kidneys. The term
"adrenal" comes from ad- (Latin, "near")
and renes (Latin, "kidney"). Similarly,
"suprarenal" is derived from supra- (Latin,
"above") and renes.
INFERIOR VENA CAVA
The inferior vena cava (or IVC), also known as the posterior vena cava,[1] is the large vein that carries de-oxygenated blood from the lower half of the body into the right atrium of the heart. It is posterior to the abdominal cavity and runs alongside of the vertebral column on its right side (i.e. it is a retroperitoneal structure). It enters the right atrium at the lower right, back side of the heart. Drainage patterns The IVC is formed by the joining of the left
and right common iliac veins and brings blood into the right atrium of the heart. It also anastomoses with the azygos vein system (which runs on the right side of the
vertebral column) and venous plexuses next to the spinal cord. The caval opening is at T8. The specific levels of the tributaries are as follows: Vein Level hepatic veins T8 inferior phrenic vein T8 suprarenal vein L1 renal veins L1 gonadal vein L2 lumbar veins L1-L5 common iliac veins L5 Because the IVC is not centrally located,
there are some asymmetries in drainage
patterns. The gonadal veins and suprarenal veins drain into the IVC on the right side, but into the renal vein on the left side, which in turn drains into the IVC. By
contrast, all the lumbar veins and hepatic veins usually drain directly into the IVC. The tributaries of Inferior vena cava can be
remembered using the mnemonic, "I Like To Rise So High", for Illiac vein (common), Lumbar vein, Testicular vein, Renal vein, Suprarenal vein and Hepatic vein.[2] Note that the vein that carries de-
oxygenated blood from the upper half of
the body is the superior vena cava . Pathologies associated with the IVC Health problems attributed to the IVC are
most often associated with it being
compressed (ruptures are rare because it
has a low intraluminal pressure). Typical sources of external pressure are an
enlarged aorta (abdominal aortic aneurysm), the gravid uterus (aortocaval compression syndrome) and abdominal maligancies, such as colorectal cancer, renal cell carcinoma and ovarian cancer. Since the inferior vena cava is primarily a
right-sided structure, unconscious pregnant
females should be turned on to their left
side (the recovery position ), to relieve pressure on it and facilitate venous return.
In rare cases, straining associated with defecation can lead to restricted blood flow through the IVC and result in syncope (fainting).[3] Occlusion of the IVC is rare, but considered
life-threatening and is an emergency. It is
associated with deep vein thrombosis, IVC filters, liver transplantation and instrumentation (e.g. catheter in the femoral vein).[4] Embryology In the embryo, the IVC and right atrium are separated by the Eustachian valve , also known in Latin as the valvula venae cavae inferioris (valve of the inferior vena cava).
In the adult, this structure typically has
totally regressed or remains as a small endocardial fold.
and right common iliac veins and brings blood into the right atrium of the heart. It also anastomoses with the azygos vein system (which runs on the right side of the
vertebral column) and venous plexuses next to the spinal cord. The caval opening is at T8. The specific levels of the tributaries are as follows: Vein Level hepatic veins T8 inferior phrenic vein T8 suprarenal vein L1 renal veins L1 gonadal vein L2 lumbar veins L1-L5 common iliac veins L5 Because the IVC is not centrally located,
there are some asymmetries in drainage
patterns. The gonadal veins and suprarenal veins drain into the IVC on the right side, but into the renal vein on the left side, which in turn drains into the IVC. By
contrast, all the lumbar veins and hepatic veins usually drain directly into the IVC. The tributaries of Inferior vena cava can be
remembered using the mnemonic, "I Like To Rise So High", for Illiac vein (common), Lumbar vein, Testicular vein, Renal vein, Suprarenal vein and Hepatic vein.[2] Note that the vein that carries de-
oxygenated blood from the upper half of
the body is the superior vena cava . Pathologies associated with the IVC Health problems attributed to the IVC are
most often associated with it being
compressed (ruptures are rare because it
has a low intraluminal pressure). Typical sources of external pressure are an
enlarged aorta (abdominal aortic aneurysm), the gravid uterus (aortocaval compression syndrome) and abdominal maligancies, such as colorectal cancer, renal cell carcinoma and ovarian cancer. Since the inferior vena cava is primarily a
right-sided structure, unconscious pregnant
females should be turned on to their left
side (the recovery position ), to relieve pressure on it and facilitate venous return.
In rare cases, straining associated with defecation can lead to restricted blood flow through the IVC and result in syncope (fainting).[3] Occlusion of the IVC is rare, but considered
life-threatening and is an emergency. It is
associated with deep vein thrombosis, IVC filters, liver transplantation and instrumentation (e.g. catheter in the femoral vein).[4] Embryology In the embryo, the IVC and right atrium are separated by the Eustachian valve , also known in Latin as the valvula venae cavae inferioris (valve of the inferior vena cava).
In the adult, this structure typically has
totally regressed or remains as a small endocardial fold.
ABDOMINAL AORTA
The abdominal aorta is the largest artery in the abdominal cavity. As part of the aorta, it is a direct continuation of the descending aorta (of the thorax). Path It begins at the level of the diaphragm, crossing it via the aortic hiatus, technically behind the diaphragm, at the vertebral
level of T12. It travels down the posterior
wall of the abdomen, anterior to the
vertebral column. It thus follows the
curvature of the lumbar vertebrae, that is,
convex anteriorly. The peak of this convexity is at the level of the third lumbar
vertebra (L3). It runs parallel to the inferior vena cava , which is located just to the right of the
abdominal aorta, and becomes smaller in
diameter as it gives off branches. This is
thought to be due to the large size of its
principal branches. At the 11th rib, the
diameter is about 25 mm; above the origin of the renal arteries, 22 mm; below the
renals, 20 mm; and at the bifurcation, 19
mm. Branches The abdominal aorta supplies blood to
much of the abdominal cavity. It begins at
T12, and usually has the following
branches: Branch Vertebra Type Paired? A/P Description inferior
phrenic T12 Parietal yes post. originates
just below
the
diaphragm,
supplying it
from below celiac Upper L1 Visceral no ant. large anterior
branch superior
mesenteric Lower
L1 Visceral no ant. large anterior
branch, arises
just below
celiac trunk middle
suprarenal L1 Visceral yes post. to adrenal gland renal In
between
L1 and
L2 Visceral yes post. large artery,
each arising
from the side
of the aorta;
supplies
corresponding kidney; arises
in the transpyloric
plane gonadal L2 Visceral yes ant. ovarian
artery in females; testicular
artery in males lumbar L1-L4 Parietal yes post. four on each
side that
supply the abdominal
wall and spinal cord inferior
mesenteric L3 Visceral no ant. large anterior
branch median
sacral L4 Parietal no post. artery arising
from the
middle of the
aorta at its
lowest part common
iliac L4 Terminal yes post. branches
(bifurcates)
to supply
blood to the lower limbs and the
pelvis, ending
the
abdominal
aorta Note that the bifurcation (union) of the inferior vena cava is at L5 and therefore below that of the bifurcation of the aorta. Contrast enhanced MRA of the abdominal aorta demonstrating normal paired arteries. 1. inferior phrenic a. 2. celiac a. 1. left gastric a. 2. splenic a. 1. short gastric arteries (6) 2. splenic arteries (6) 3. left gastroepiploic a. 3. hepatic a. 1. cystic a. 2. right gastric a. 3. gastroduodenal a. 1. right gastroepiploic a. 2. superior pancreaticoduodenal a. 4. right hepatic a. 5. left hepatic a. 1. superior mesenteric a. 1. jejunal and ileal arteries 2. inferior pancreaticoduodenal a. 3. middle colic a. 4. right colic a. 5. ileocolic a 1. anterior cecal a. 2. posterior cecal a. – appendicular a. 3. ileal a. 4. colic a. 1. middle suprarenal a. 2. renal a. 3. testicular or ovarian a. 1. four lumbar arteries 1. inferior mesenteric a. 1. left colic a. 2. sigmoid arteries (2 or 3) 3. superior rectal a. 1. median sacral a. 1. common iliac a. 1. external iliac a. 2. internal iliac a. Relations The abdominal aorta lies slightly to the left
of the midline of the body. It is covered,
anteriorly, by the lesser omentum and stomach, behind which are the branches of the celiac artery and the celiac plexus;
below these, by the lienal vein(splenic artery), the pancreas, the left renal vein, the inferior part of the duodenum, the mesentery, and aortic plexus. Posteriorly, it is separated from the lumbar
vertebræ and intervertebral fibrocartilages
by the anterior longitudinal ligament and left lumbar veins. On the right side it is in relation above
with the azygos vein , cisterna chyli, thoracic duct, and the right crus of the diaphragm—the last separating it from the
upper part of the inferior vena cava , and from the right celiac ganglion; the inferior
vena cava is in contact with the aorta
below. On the left side are the left crus of the
diaphragm, the left celiac ganglion, the
ascending part of the duodenum, and some
coils of the small intestine. Relationship with inferior vena cava The abominal aorta's venous counterpart,
the inferior vena cava (IVC), travels parallel to it on its right side. Above the level of the umbilicus, the aorta is somewhat posterior to the IVC,
sending the right renal artery travelling behind it. The IVC likewise sends its
opposite side counterpart, the left renal vein, crossing in front of the aorta. Below the level of the umbilicus, the
situation is generally reversed, with the
aorta sending its right common iliac artery to cross its opposite side counterpart (the left common iliac vein) anteriorly. Collateral circulation The collateral circulation would be carried
on by the anastomoses between the internal thoracic artery and the inferior epigastric artery; by the free communication between the superior and
inferior mesenterics, if the ligature were
placed between these vessels; or by the
anastomosis between the inferior mesenteric artery and the internal pudendal artery, when (as is more common) the point of ligature is below the
origin of the inferior mesenteric artery; and possibly by the anastomoses of the lumbar arteries with the branches of the internal iliac artery.
level of T12. It travels down the posterior
wall of the abdomen, anterior to the
vertebral column. It thus follows the
curvature of the lumbar vertebrae, that is,
convex anteriorly. The peak of this convexity is at the level of the third lumbar
vertebra (L3). It runs parallel to the inferior vena cava , which is located just to the right of the
abdominal aorta, and becomes smaller in
diameter as it gives off branches. This is
thought to be due to the large size of its
principal branches. At the 11th rib, the
diameter is about 25 mm; above the origin of the renal arteries, 22 mm; below the
renals, 20 mm; and at the bifurcation, 19
mm. Branches The abdominal aorta supplies blood to
much of the abdominal cavity. It begins at
T12, and usually has the following
branches: Branch Vertebra Type Paired? A/P Description inferior
phrenic T12 Parietal yes post. originates
just below
the
diaphragm,
supplying it
from below celiac Upper L1 Visceral no ant. large anterior
branch superior
mesenteric Lower
L1 Visceral no ant. large anterior
branch, arises
just below
celiac trunk middle
suprarenal L1 Visceral yes post. to adrenal gland renal In
between
L1 and
L2 Visceral yes post. large artery,
each arising
from the side
of the aorta;
supplies
corresponding kidney; arises
in the transpyloric
plane gonadal L2 Visceral yes ant. ovarian
artery in females; testicular
artery in males lumbar L1-L4 Parietal yes post. four on each
side that
supply the abdominal
wall and spinal cord inferior
mesenteric L3 Visceral no ant. large anterior
branch median
sacral L4 Parietal no post. artery arising
from the
middle of the
aorta at its
lowest part common
iliac L4 Terminal yes post. branches
(bifurcates)
to supply
blood to the lower limbs and the
pelvis, ending
the
abdominal
aorta Note that the bifurcation (union) of the inferior vena cava is at L5 and therefore below that of the bifurcation of the aorta. Contrast enhanced MRA of the abdominal aorta demonstrating normal paired arteries. 1. inferior phrenic a. 2. celiac a. 1. left gastric a. 2. splenic a. 1. short gastric arteries (6) 2. splenic arteries (6) 3. left gastroepiploic a. 3. hepatic a. 1. cystic a. 2. right gastric a. 3. gastroduodenal a. 1. right gastroepiploic a. 2. superior pancreaticoduodenal a. 4. right hepatic a. 5. left hepatic a. 1. superior mesenteric a. 1. jejunal and ileal arteries 2. inferior pancreaticoduodenal a. 3. middle colic a. 4. right colic a. 5. ileocolic a 1. anterior cecal a. 2. posterior cecal a. – appendicular a. 3. ileal a. 4. colic a. 1. middle suprarenal a. 2. renal a. 3. testicular or ovarian a. 1. four lumbar arteries 1. inferior mesenteric a. 1. left colic a. 2. sigmoid arteries (2 or 3) 3. superior rectal a. 1. median sacral a. 1. common iliac a. 1. external iliac a. 2. internal iliac a. Relations The abdominal aorta lies slightly to the left
of the midline of the body. It is covered,
anteriorly, by the lesser omentum and stomach, behind which are the branches of the celiac artery and the celiac plexus;
below these, by the lienal vein(splenic artery), the pancreas, the left renal vein, the inferior part of the duodenum, the mesentery, and aortic plexus. Posteriorly, it is separated from the lumbar
vertebræ and intervertebral fibrocartilages
by the anterior longitudinal ligament and left lumbar veins. On the right side it is in relation above
with the azygos vein , cisterna chyli, thoracic duct, and the right crus of the diaphragm—the last separating it from the
upper part of the inferior vena cava , and from the right celiac ganglion; the inferior
vena cava is in contact with the aorta
below. On the left side are the left crus of the
diaphragm, the left celiac ganglion, the
ascending part of the duodenum, and some
coils of the small intestine. Relationship with inferior vena cava The abominal aorta's venous counterpart,
the inferior vena cava (IVC), travels parallel to it on its right side. Above the level of the umbilicus, the aorta is somewhat posterior to the IVC,
sending the right renal artery travelling behind it. The IVC likewise sends its
opposite side counterpart, the left renal vein, crossing in front of the aorta. Below the level of the umbilicus, the
situation is generally reversed, with the
aorta sending its right common iliac artery to cross its opposite side counterpart (the left common iliac vein) anteriorly. Collateral circulation The collateral circulation would be carried
on by the anastomoses between the internal thoracic artery and the inferior epigastric artery; by the free communication between the superior and
inferior mesenterics, if the ligature were
placed between these vessels; or by the
anastomosis between the inferior mesenteric artery and the internal pudendal artery, when (as is more common) the point of ligature is below the
origin of the inferior mesenteric artery; and possibly by the anastomoses of the lumbar arteries with the branches of the internal iliac artery.
ILIAC CREST
The crest of the ilium (or iliac crest) is the superior border of the wing of ilium and the superolateral margin of the greater pelvis. Anatomy The iliac crest stretches posteriorly from
the anterior superior iliac spine (ASIS) to the posterior superior iliac spine (PSIS). Behind the ASIS, it divides into an outer
and inner lip separated by the
intermediate zone. The outer lip bulges laterally into the iliac tubercle. [1]Palpable in its entire length, the crest is convex
superiorly but is sinuously curved, being
concave inward in front, concave outward behind. [2] It is thinner at the center than at the
extremities. Muscles To the external lip are attached the Tensor fasciae latae, Obliquus externus abdominis, and Latissimus dorsi, and along its whole length the fascia lata; to the intermediate line the Obliquus internus abdominis. To the internal lip, the iliac fascia, the Transversus abdominis, Quadratus lumborum, Sacrospinalis, and Iliacus. Abdominal external oblique muscle Abdominal internal oblique muscle Transversus abdominis muscle Quadratus lumborum muscle Erector spinae Iliocostalis pars lumborum Longissimus pars thoracis[3] Latissimus dorsi Tensor fasciae latae Iliacus muscle Fascia lata Iliac fascia Transverse fascia Embryology The iliac crest is derived from endochondral bone. Clinical significance The iliac crest has a large amount of red bone marrow, and thus it is the site of bone marrow harvests (from both sides) to
collect the stem cells used in bone marrow transplantation. The iliac crest is also considered the most ideal donor site for
bone grafting when a large quantity of
bone is needed. For example, oral surgeons
will often use iliac crest bone to fill in large
osseous defects of the oral cavity caused
from severe periodontal disease, excess bone resorption following tooth loss, or trauma [4] The top of the iliac crests also marks the level of the fourth lumbar
vertebral body (L4), above or below which lumbar puncture may be performed.
the anterior superior iliac spine (ASIS) to the posterior superior iliac spine (PSIS). Behind the ASIS, it divides into an outer
and inner lip separated by the
intermediate zone. The outer lip bulges laterally into the iliac tubercle. [1]Palpable in its entire length, the crest is convex
superiorly but is sinuously curved, being
concave inward in front, concave outward behind. [2] It is thinner at the center than at the
extremities. Muscles To the external lip are attached the Tensor fasciae latae, Obliquus externus abdominis, and Latissimus dorsi, and along its whole length the fascia lata; to the intermediate line the Obliquus internus abdominis. To the internal lip, the iliac fascia, the Transversus abdominis, Quadratus lumborum, Sacrospinalis, and Iliacus. Abdominal external oblique muscle Abdominal internal oblique muscle Transversus abdominis muscle Quadratus lumborum muscle Erector spinae Iliocostalis pars lumborum Longissimus pars thoracis[3] Latissimus dorsi Tensor fasciae latae Iliacus muscle Fascia lata Iliac fascia Transverse fascia Embryology The iliac crest is derived from endochondral bone. Clinical significance The iliac crest has a large amount of red bone marrow, and thus it is the site of bone marrow harvests (from both sides) to
collect the stem cells used in bone marrow transplantation. The iliac crest is also considered the most ideal donor site for
bone grafting when a large quantity of
bone is needed. For example, oral surgeons
will often use iliac crest bone to fill in large
osseous defects of the oral cavity caused
from severe periodontal disease, excess bone resorption following tooth loss, or trauma [4] The top of the iliac crests also marks the level of the fourth lumbar
vertebral body (L4), above or below which lumbar puncture may be performed.
PSOAS MAJOR MUSCLE
The psoas major is a long fusiform muscle located on the side of the lumbar region of
the vertebral column and brim of the lesser pelvis. It joins the iliacus muscle to form the iliopsoas. In less than 50 percent of human subjects[1] the psoas major is accompanied by the psoas minor. In mice, it is mostly a fast-twitching, type II muscle,[2] while in human it combines slow and fast-twitching fibers. [3] Location Origin The psoas (\ˈsō-əs\) major is divided into a
superficial and deep part. The deep part
originates from the transverse processes of lumbar vertebrae I-V. The superficial part originates from the lateral surfaces of the
last thoracic vertebra, lumbar vertebrae I- IV, and from neighboring invertebral discs. The lumbar plexus lies between the two layers.[1] Insertion Joined by the iliacus, psoas major forms the iliopsoas which is surrounded by the iliac fascia. The iliopsoas runs across the iliopubic eminence through the muscular lacuna to its insertion on the lesser trochanter of the femur. The iliopectineal bursa separates the bone from the muscle at the level of the iliopubic eminence. The
iliac subtendinous bursa lies between the
lesser trochanter and the attachment of the iliopsoas.[1] Innervation Innervation of the psoas major is through
the anterior rami of L1 to L3 Function As part of the iliopsoas, psoas major
contributes to flexion and external rotation in the hip joint. On the lumbar spine,
unilateral contraction bends the trunk
laterally, while bilateral contraction raises the trunk from its supine position.[4] It forms part of a group of muscles called
the hip flexors, whose action is primarily to lift the upper leg towards the body when
the body is fixed or to pull the body
towards the leg when the leg is fixed. For example, when doing a situp that
brings the torso (including the lower back)
away from the ground and towards the
front of the leg, the hip flexors (including
the iliopsoas) will flex the spine upon the
pelvis. Due to the frontal attachment on the
vertebrae, rotation of the spine will stretch
the psoas. Tightness of the psoas can result in lower
back pain by compressing the lumbar discs.
the vertebral column and brim of the lesser pelvis. It joins the iliacus muscle to form the iliopsoas. In less than 50 percent of human subjects[1] the psoas major is accompanied by the psoas minor. In mice, it is mostly a fast-twitching, type II muscle,[2] while in human it combines slow and fast-twitching fibers. [3] Location Origin The psoas (\ˈsō-əs\) major is divided into a
superficial and deep part. The deep part
originates from the transverse processes of lumbar vertebrae I-V. The superficial part originates from the lateral surfaces of the
last thoracic vertebra, lumbar vertebrae I- IV, and from neighboring invertebral discs. The lumbar plexus lies between the two layers.[1] Insertion Joined by the iliacus, psoas major forms the iliopsoas which is surrounded by the iliac fascia. The iliopsoas runs across the iliopubic eminence through the muscular lacuna to its insertion on the lesser trochanter of the femur. The iliopectineal bursa separates the bone from the muscle at the level of the iliopubic eminence. The
iliac subtendinous bursa lies between the
lesser trochanter and the attachment of the iliopsoas.[1] Innervation Innervation of the psoas major is through
the anterior rami of L1 to L3 Function As part of the iliopsoas, psoas major
contributes to flexion and external rotation in the hip joint. On the lumbar spine,
unilateral contraction bends the trunk
laterally, while bilateral contraction raises the trunk from its supine position.[4] It forms part of a group of muscles called
the hip flexors, whose action is primarily to lift the upper leg towards the body when
the body is fixed or to pull the body
towards the leg when the leg is fixed. For example, when doing a situp that
brings the torso (including the lower back)
away from the ground and towards the
front of the leg, the hip flexors (including
the iliopsoas) will flex the spine upon the
pelvis. Due to the frontal attachment on the
vertebrae, rotation of the spine will stretch
the psoas. Tightness of the psoas can result in lower
back pain by compressing the lumbar discs.
STRUCTURE OF THE KIDNEY
1. Renal pyramid • 2. Interlobular artery • 3. Renal artery • 4. Renal vein 5. Renal hilum • 6. Renal pelvis • 7. Ureter • 8. Minor calyx • 9. Renal capsule • 10. Inferior renal capsule • 11. Superior renal capsule • 12. Interlobular vein • 13. Nephron • 14. Minor calyx • 15. Major calyx • 16. Renal papilla • 17. Renal column The kidney has a bean-shaped structure;
each kidney has a convex and concave
surface. The concave surface, the renal hilum, is the point at which the renal artery enters the organ, and the renal vein and ureter leave. The kidney is surrounded by tough fibrous tissue, the renal capsule, which is itself surrounded by perinephric fat, renal fascia (of Gerota) and paranephric fat. The anterior (front) border of these tissues is the peritoneum, while the posterior (rear) border is the transversalis fascia. The superior border of the right kidney is
adjacent to the liver; and the spleen, for the left kidney. Therefore, both move
down on inhalation. The kidney is approximately 11–14 cm in
length, 6 cm wide and 4 cm thick. The substance, or parenchyma, of the kidney is divided into two major
structures: superficial is the renal cortex and deep is the renal medulla. Grossly, these structures take the shape of 8 to 18
cone-shaped renal lobes, each containing renal cortex surrounding a portion of
medulla called a renal pyramid (of Malpighi).[5] Between the renal pyramids are projections of cortex called renal columns (of Bertin). Nephrons, the urine- producing functional structures of the
kidney, span the cortex and medulla. The
initial filtering portion of a nephron is the renal corpuscle, located in the cortex, which is followed by a renal tubule that passes from the cortex deep into the
medullary pyramids. Part of the renal
cortex, a medullary ray is a collection of renal tubules that drain into a single collecting duct. The tip, or papilla, of each pyramid empties urine into a minor calyx; minor calyces empty into major calyces, and major calyces empty into the renal pelvis, which becomes the ureter. Blood supply 3D-rendered computed tomography, showing renal arteries and veins. The kidneys receive blood from the renal arteries, left and right, which branch directly from the abdominal aorta. Despite their relatively small size, the kidneys
receive approximately 20% of the cardiac output.[5] Each renal artery branches into segmental
arteries, dividing further into interlobar arteries which penetrate the renal capsule and extend through the renal columns
between the renal pyramids. The
interlobar arteries then supply blood to the arcuate arteries that run through the boundary of the cortex and the medulla.
Each arcuate artery supplies several interlobular arteries that feed into the afferent arterioles that supply the glomeruli. The interstitum (or interstitium) is the functional space in the kidney beneath the
individual filters (glomeruli) which are rich
in blood vessels. The interstitum absorbs fluid recovered from urine. Various conditions can lead to scarring and congestion of this area, which can cause kidney dysfunction and failure. After filtration occurs the blood moves
through a small network of venules that
converge into interlobular veins. As with
the arteriole distribution the veins follow
the same pattern, the interlobular provide
blood to the arcuate veins then back to the interlobar veins which come to form the
renal vein exiting the kidney for
transfusion for blood. Histology Microscopic photograph of the renal medulla. Microscopic photograph of the renal cortex. Renal histology studies the structure of the kidney as viewed under a microscope. Various distinct cell types occur in the kidney, including: Kidney glomerulus parietal cell Kidney glomerulus podocyte Kidney proximal tubule brush border cell Loop of Henle thin segment cell Thick ascending limb cell Kidney distal tubule cell Kidney collecting duct cell Interstitial kidney cells Innervation The kidney and nervous system communicate via the renal plexus, whose fibers course along the renal arteries to reach the kidney.[7] Input from the sympathetic nervous system triggers vasoconstriction in the kidney, thereby reducing renal blood flow.[7] The kidney is not thought to receive input from the parasympathetic nervous system .[7] Sensory input from the kidney travels to
the T10-11 levels of the spinal cord and is sensed in the corresponding dermatome.[7] Thus, pain in the flank region may be referred from the kidney. [7] Functions Main article: Renal physiology The kidney participates in whole-body homeostasis, regulating acid-base balance, electrolyte concentrations, extracellular fluid volume, and regulation of blood pressure. The kidney accomplishes these homeostatic functions both independently
and in concert with other organs,
particularly those of the endocrine system. Various endocrine hormones coordinate
these endocrine functions; these include renin, angiotensin II, aldosterone, antidiuretic hormone, and atrial natriuretic peptide, among others. Many of the kidney's functions are
accomplished by relatively simple
mechanisms of filtration, reabsorption, and
secretion, which take place in the nephron. Filtration, which takes place at the renal corpuscle, is the process by which cells and large proteins are filtered from the blood
to make an ultrafiltrate that eventually
becomes urine. The kidney generates 180
liters of filtrate a day, while reabsorbing a
large percentage, allowing for the
generation of only approximately 2 liters of urine. Reabsorption is the transport of
molecules from this ultrafiltrate and into
the blood. Secretion is the reverse process,
in which molecules are transported in the
opposite direction, from the blood into the
urine. Excretion of wastes The kidneys excrete a variety of waste
products produced by metabolism. These include the nitrogenous wastes called
"urea", from protein catabolism, as well as uric acid, from nucleic acid metabolism. Formation of urine is also the function of
the kidney. Acid-base homeostasis Main article: Acid-base homeostasis Two organ systems, the kidneys and lungs,
maintain acid-base homeostasis, which is
the maintenance of pH around a relatively stable value. The lungs contribute to acid-
base homeostasis by regulating bicarbonate (HCO3-) concentration. The kidneys have two very important roles in
maintaining the acid-base balance: to
reabsorb bicarbonate from urine, and to
excrete hydrogen ions into urine Osmolality regulation Any significant rise in plasma osmolality is detected by the hypothalamus, which communicates directly with the posterior pituitary gland. An increase in osmolality causes the gland to secrete antidiuretic hormone (ADH), resulting in water reabsorption by the kidney and an increase
in urine concentration. The two factors
work together to return the plasma
osmolality to its normal levels. ADH binds to principal cells in the collecting
duct that translocate aquaporins to the
membrane, allowing water to leave the
normally impermeable membrane and be
reabsorbed into the body by the vasa recta,
thus increasing the plasma volume of the body. There are two systems that create a
hyperosmotic medulla and thus increase
the body plasma volume: Urea recycling
and the 'single effect.' Urea is usually excreted as a waste product
from the kidneys. However, when plasma
blood volume is low and ADH is released
the aquaporins that are opened are also
permeable to urea. This allows urea to
leave the collecting duct into the medulla creating a hyperosmotic solution that
'attracts' water. Urea can then re-enter the
nephron and be excreted or recycled again
depending on whether ADH is still present
or not. The 'Single effect' describes the fact that
the ascending thick limb of the loop of
Henle is not permeable to water but is
permeable to NaCl. This allows for a countercurrent exchange system whereby the medulla becomes increasingly
concentrated, but at the same time setting
up an osmotic gradient for water to follow
should the aquaporins of the collecting duct
be opened by ADH. Blood pressure regulation Main articles: Blood pressure regulation and Renin-angiotensin system Long-term regulation of blood pressure predominantly depends upon the kidney.
This primarily occurs through maintenance
of the extracellular fluid compartment, the size of which depends on the plasma sodium concentration. Although the kidney cannot directly sense blood pressure,
changes in the delivery of sodium and chloride to the distal part of the nephron alter the kidney's secretion of the enzyme renin. When the extracellular fluid compartment is expanded and blood
pressure is high, the delivery of these ions
is increased and renin secretion is
decreased. Similarly, when the
extracellular fluid compartment is
contracted and blood pressure is low, sodium and chloride delivery is decreased
and renin secretion is increased in
response. Renin is the first in a series of important
chemical messengers that comprise the renin-angiotensin system. Changes in renin ultimately alter the output of this system,
principally the hormones angiotensin II and aldosterone. Each hormone acts via multiple mechanisms, but both increase the
kidney's absorption of sodium chloride,
thereby expanding the extracellular fluid
compartment and raising blood pressure.
When renin levels are elevated, the
concentrations of angiotensin II and aldosterone increase, leading to increased
sodium chloride reabsorption, expansion of
the extracellular fluid compartment, and an
increase in blood pressure. Conversely,
when renin levels are low, angiotensin II
and aldosterone levels decrease, contracting the extracellular fluid
compartment, and decreasing blood
pressure. Hormone secretion The kidneys secrete a variety of hormones, including erythropoietin, and the enzyme renin. Erythropoietin is released in response to hypoxia (low levels of oxygen at tissue level) in the renal circulation. It
stimulates erythropoiesis (production of red blood cells) in the bone marrow. Calcitriol, the activated form of vitamin D, promotes intestinal absorption of calcium and the renal reabsorption of phosphate. Part of the renin-angiotensin-aldosterone system, renin is an enzyme involved in the regulation of aldosterone levels. Development Main article: Kidney development The mammalian kidney develops from intermediate mesoderm. Kidney development, also called nephrogenesis, proceeds through a series of three
successive phases, each marked by the
development of a more advanced pair of
kidneys: the pronephros, mesonephros, and metanephros.[8] Evolutionary adaptation Kidneys of various animals show evidence
of evolutionary adaptation and have long been studied in ecophysiology and comparative physiology . Kidney morphology, often indexed as the relative
medullary thickness, is associated with
habitat aridity among species of mammals. [9] Etymology Medical terms related to the kidneys
commonly use terms such as renal and the
prefix nephro-. The adjective renal, meaning related to the kidney, is from the Latin rēnēs, meaning kidneys; the prefix nephro- is from the Ancient Greek word for kidney, nephros (νεφρός).[10] For example, surgical removal of the kidney is a nephrectomy, while a reduction in kidney function is called renal dysfunction. Diseases and disorders Main article: Nephropathy Congenital Congenital hydronephrosis Congenital obstruction of urinary tract Duplex kidneys, or double kidneys, occur
in approximately 1% of the population.
This occurrence normally causes no
complications, but can occasionally cause urine infections.[11][12] Duplicated ureter occurs in approximately one in 100 live births Horseshoe kidney occurs in approximately one in 400 live births Polycystic kidney disease Autosomal dominant polycystic
kidney disease afflicts patients later in life. Approximately one in 1000
people will develop this condition Autosomal recessive polycystic
kidney disease is far less common, but more severe, than the dominant
condition. It is apparent in utero or at
birth. Renal agenesis. Failure of one kidney to form occurs in approximately one in 750
live births. Failure of both kidneys to
form is invariably fatal. Renal dysplasia Unilateral small kidney Multicystic dysplastic kidney occurs in approximately one in every 2400 live
births Ureteropelvic Junction Obstruction or
UPJO; although most cases appear
congenital, some appear to be an acquired condition
each kidney has a convex and concave
surface. The concave surface, the renal hilum, is the point at which the renal artery enters the organ, and the renal vein and ureter leave. The kidney is surrounded by tough fibrous tissue, the renal capsule, which is itself surrounded by perinephric fat, renal fascia (of Gerota) and paranephric fat. The anterior (front) border of these tissues is the peritoneum, while the posterior (rear) border is the transversalis fascia. The superior border of the right kidney is
adjacent to the liver; and the spleen, for the left kidney. Therefore, both move
down on inhalation. The kidney is approximately 11–14 cm in
length, 6 cm wide and 4 cm thick. The substance, or parenchyma, of the kidney is divided into two major
structures: superficial is the renal cortex and deep is the renal medulla. Grossly, these structures take the shape of 8 to 18
cone-shaped renal lobes, each containing renal cortex surrounding a portion of
medulla called a renal pyramid (of Malpighi).[5] Between the renal pyramids are projections of cortex called renal columns (of Bertin). Nephrons, the urine- producing functional structures of the
kidney, span the cortex and medulla. The
initial filtering portion of a nephron is the renal corpuscle, located in the cortex, which is followed by a renal tubule that passes from the cortex deep into the
medullary pyramids. Part of the renal
cortex, a medullary ray is a collection of renal tubules that drain into a single collecting duct. The tip, or papilla, of each pyramid empties urine into a minor calyx; minor calyces empty into major calyces, and major calyces empty into the renal pelvis, which becomes the ureter. Blood supply 3D-rendered computed tomography, showing renal arteries and veins. The kidneys receive blood from the renal arteries, left and right, which branch directly from the abdominal aorta. Despite their relatively small size, the kidneys
receive approximately 20% of the cardiac output.[5] Each renal artery branches into segmental
arteries, dividing further into interlobar arteries which penetrate the renal capsule and extend through the renal columns
between the renal pyramids. The
interlobar arteries then supply blood to the arcuate arteries that run through the boundary of the cortex and the medulla.
Each arcuate artery supplies several interlobular arteries that feed into the afferent arterioles that supply the glomeruli. The interstitum (or interstitium) is the functional space in the kidney beneath the
individual filters (glomeruli) which are rich
in blood vessels. The interstitum absorbs fluid recovered from urine. Various conditions can lead to scarring and congestion of this area, which can cause kidney dysfunction and failure. After filtration occurs the blood moves
through a small network of venules that
converge into interlobular veins. As with
the arteriole distribution the veins follow
the same pattern, the interlobular provide
blood to the arcuate veins then back to the interlobar veins which come to form the
renal vein exiting the kidney for
transfusion for blood. Histology Microscopic photograph of the renal medulla. Microscopic photograph of the renal cortex. Renal histology studies the structure of the kidney as viewed under a microscope. Various distinct cell types occur in the kidney, including: Kidney glomerulus parietal cell Kidney glomerulus podocyte Kidney proximal tubule brush border cell Loop of Henle thin segment cell Thick ascending limb cell Kidney distal tubule cell Kidney collecting duct cell Interstitial kidney cells Innervation The kidney and nervous system communicate via the renal plexus, whose fibers course along the renal arteries to reach the kidney.[7] Input from the sympathetic nervous system triggers vasoconstriction in the kidney, thereby reducing renal blood flow.[7] The kidney is not thought to receive input from the parasympathetic nervous system .[7] Sensory input from the kidney travels to
the T10-11 levels of the spinal cord and is sensed in the corresponding dermatome.[7] Thus, pain in the flank region may be referred from the kidney. [7] Functions Main article: Renal physiology The kidney participates in whole-body homeostasis, regulating acid-base balance, electrolyte concentrations, extracellular fluid volume, and regulation of blood pressure. The kidney accomplishes these homeostatic functions both independently
and in concert with other organs,
particularly those of the endocrine system. Various endocrine hormones coordinate
these endocrine functions; these include renin, angiotensin II, aldosterone, antidiuretic hormone, and atrial natriuretic peptide, among others. Many of the kidney's functions are
accomplished by relatively simple
mechanisms of filtration, reabsorption, and
secretion, which take place in the nephron. Filtration, which takes place at the renal corpuscle, is the process by which cells and large proteins are filtered from the blood
to make an ultrafiltrate that eventually
becomes urine. The kidney generates 180
liters of filtrate a day, while reabsorbing a
large percentage, allowing for the
generation of only approximately 2 liters of urine. Reabsorption is the transport of
molecules from this ultrafiltrate and into
the blood. Secretion is the reverse process,
in which molecules are transported in the
opposite direction, from the blood into the
urine. Excretion of wastes The kidneys excrete a variety of waste
products produced by metabolism. These include the nitrogenous wastes called
"urea", from protein catabolism, as well as uric acid, from nucleic acid metabolism. Formation of urine is also the function of
the kidney. Acid-base homeostasis Main article: Acid-base homeostasis Two organ systems, the kidneys and lungs,
maintain acid-base homeostasis, which is
the maintenance of pH around a relatively stable value. The lungs contribute to acid-
base homeostasis by regulating bicarbonate (HCO3-) concentration. The kidneys have two very important roles in
maintaining the acid-base balance: to
reabsorb bicarbonate from urine, and to
excrete hydrogen ions into urine Osmolality regulation Any significant rise in plasma osmolality is detected by the hypothalamus, which communicates directly with the posterior pituitary gland. An increase in osmolality causes the gland to secrete antidiuretic hormone (ADH), resulting in water reabsorption by the kidney and an increase
in urine concentration. The two factors
work together to return the plasma
osmolality to its normal levels. ADH binds to principal cells in the collecting
duct that translocate aquaporins to the
membrane, allowing water to leave the
normally impermeable membrane and be
reabsorbed into the body by the vasa recta,
thus increasing the plasma volume of the body. There are two systems that create a
hyperosmotic medulla and thus increase
the body plasma volume: Urea recycling
and the 'single effect.' Urea is usually excreted as a waste product
from the kidneys. However, when plasma
blood volume is low and ADH is released
the aquaporins that are opened are also
permeable to urea. This allows urea to
leave the collecting duct into the medulla creating a hyperosmotic solution that
'attracts' water. Urea can then re-enter the
nephron and be excreted or recycled again
depending on whether ADH is still present
or not. The 'Single effect' describes the fact that
the ascending thick limb of the loop of
Henle is not permeable to water but is
permeable to NaCl. This allows for a countercurrent exchange system whereby the medulla becomes increasingly
concentrated, but at the same time setting
up an osmotic gradient for water to follow
should the aquaporins of the collecting duct
be opened by ADH. Blood pressure regulation Main articles: Blood pressure regulation and Renin-angiotensin system Long-term regulation of blood pressure predominantly depends upon the kidney.
This primarily occurs through maintenance
of the extracellular fluid compartment, the size of which depends on the plasma sodium concentration. Although the kidney cannot directly sense blood pressure,
changes in the delivery of sodium and chloride to the distal part of the nephron alter the kidney's secretion of the enzyme renin. When the extracellular fluid compartment is expanded and blood
pressure is high, the delivery of these ions
is increased and renin secretion is
decreased. Similarly, when the
extracellular fluid compartment is
contracted and blood pressure is low, sodium and chloride delivery is decreased
and renin secretion is increased in
response. Renin is the first in a series of important
chemical messengers that comprise the renin-angiotensin system. Changes in renin ultimately alter the output of this system,
principally the hormones angiotensin II and aldosterone. Each hormone acts via multiple mechanisms, but both increase the
kidney's absorption of sodium chloride,
thereby expanding the extracellular fluid
compartment and raising blood pressure.
When renin levels are elevated, the
concentrations of angiotensin II and aldosterone increase, leading to increased
sodium chloride reabsorption, expansion of
the extracellular fluid compartment, and an
increase in blood pressure. Conversely,
when renin levels are low, angiotensin II
and aldosterone levels decrease, contracting the extracellular fluid
compartment, and decreasing blood
pressure. Hormone secretion The kidneys secrete a variety of hormones, including erythropoietin, and the enzyme renin. Erythropoietin is released in response to hypoxia (low levels of oxygen at tissue level) in the renal circulation. It
stimulates erythropoiesis (production of red blood cells) in the bone marrow. Calcitriol, the activated form of vitamin D, promotes intestinal absorption of calcium and the renal reabsorption of phosphate. Part of the renin-angiotensin-aldosterone system, renin is an enzyme involved in the regulation of aldosterone levels. Development Main article: Kidney development The mammalian kidney develops from intermediate mesoderm. Kidney development, also called nephrogenesis, proceeds through a series of three
successive phases, each marked by the
development of a more advanced pair of
kidneys: the pronephros, mesonephros, and metanephros.[8] Evolutionary adaptation Kidneys of various animals show evidence
of evolutionary adaptation and have long been studied in ecophysiology and comparative physiology . Kidney morphology, often indexed as the relative
medullary thickness, is associated with
habitat aridity among species of mammals. [9] Etymology Medical terms related to the kidneys
commonly use terms such as renal and the
prefix nephro-. The adjective renal, meaning related to the kidney, is from the Latin rēnēs, meaning kidneys; the prefix nephro- is from the Ancient Greek word for kidney, nephros (νεφρός).[10] For example, surgical removal of the kidney is a nephrectomy, while a reduction in kidney function is called renal dysfunction. Diseases and disorders Main article: Nephropathy Congenital Congenital hydronephrosis Congenital obstruction of urinary tract Duplex kidneys, or double kidneys, occur
in approximately 1% of the population.
This occurrence normally causes no
complications, but can occasionally cause urine infections.[11][12] Duplicated ureter occurs in approximately one in 100 live births Horseshoe kidney occurs in approximately one in 400 live births Polycystic kidney disease Autosomal dominant polycystic
kidney disease afflicts patients later in life. Approximately one in 1000
people will develop this condition Autosomal recessive polycystic
kidney disease is far less common, but more severe, than the dominant
condition. It is apparent in utero or at
birth. Renal agenesis. Failure of one kidney to form occurs in approximately one in 750
live births. Failure of both kidneys to
form is invariably fatal. Renal dysplasia Unilateral small kidney Multicystic dysplastic kidney occurs in approximately one in every 2400 live
births Ureteropelvic Junction Obstruction or
UPJO; although most cases appear
congenital, some appear to be an acquired condition
RENAL MEDULLA
The renal medulla is the innermost part of the kidney. The renal medulla is split up into a number of sections, known as the renal pyramids. Blood enters into the kidney via the renal artery, which then
splits up to form the arcuate arterioles. The arcuate arterioles each in turn branch into interlobular arterioles, which finally reach
the glomeruli. At the glomerulus the blood reaches a highly disfavourable pressure
gradient and a large exchange surface
area, which forces the serum portion of the blood out of the vessel into the renal
tubules. Flow continues through the renal
tubules, including the proximal tubule, the Loop of Henle, and finally leaves the kidney by means of the collecting duct, leading to the renal ureter. The renal medulla (latin renes medulla = kidney middle) contains the structures of
the nephrons responsible for maintaining the salt and water balance of the blood.
These structures include the vasa rectae
(both spuria and vera), the venulae rectae,
the medullary capillary plexus, the loop of Henle, and the collecting tubule.[1] The renal medulla is hypertonic to the filtrate
in the nephron and aids in the reabsorption
of water. The portion of blood that is passed through
the glomerulus is the plasma, not serum.
Blood is filtered in the glomerulus, and is
not a selective process. Ions such as
sodium, chloride, potassium, and calcium
are easily filtered, as is glucose, because filtration is based on size. Proteins are not
passed through the glomerular filter
because of their large size, and do not
appear in the filtrate or urine unless a
disease process has affected the
glomerular capsule or the proximal and distule tubules of the nephron.
splits up to form the arcuate arterioles. The arcuate arterioles each in turn branch into interlobular arterioles, which finally reach
the glomeruli. At the glomerulus the blood reaches a highly disfavourable pressure
gradient and a large exchange surface
area, which forces the serum portion of the blood out of the vessel into the renal
tubules. Flow continues through the renal
tubules, including the proximal tubule, the Loop of Henle, and finally leaves the kidney by means of the collecting duct, leading to the renal ureter. The renal medulla (latin renes medulla = kidney middle) contains the structures of
the nephrons responsible for maintaining the salt and water balance of the blood.
These structures include the vasa rectae
(both spuria and vera), the venulae rectae,
the medullary capillary plexus, the loop of Henle, and the collecting tubule.[1] The renal medulla is hypertonic to the filtrate
in the nephron and aids in the reabsorption
of water. The portion of blood that is passed through
the glomerulus is the plasma, not serum.
Blood is filtered in the glomerulus, and is
not a selective process. Ions such as
sodium, chloride, potassium, and calcium
are easily filtered, as is glucose, because filtration is based on size. Proteins are not
passed through the glomerular filter
because of their large size, and do not
appear in the filtrate or urine unless a
disease process has affected the
glomerular capsule or the proximal and distule tubules of the nephron.
RENAL SINUS
Renal sinus The renal sinus is a cavity within the kidney which is occupied by the renal pelvis, renal calyces, blood vessels, nerves and fat.
RENAL LOBE
The renal lobe is a portion of a kidney consisting of a renal pyramid and the renal cortex above it. [1] It is visible without a microscope, though it is easier to see in humans than in other
animals. It is composed of many renal lobules, which are not visible without a
microscope.
animals. It is composed of many renal lobules, which are not visible without a
microscope.
RENAL COLUMN
The renal column (or Bertin column, or column of Bertin) is a medullary extension of the renal cortex in between the renal pyramids. It allows the cortex to be better anchored. Each column consists of lines of blood
vessels and urinary tubes and a fibrous
material.
vessels and urinary tubes and a fibrous
material.
MINOR CALYX
The minor calyx, in the kidney, surrounds the apex of the renal pyramids. Urine formed in the kidney passes through a papilla at the apex into the minor calyx
then into the major calyx. Peristalsis of the smooth muscle originating in pace-maker cells originating
in the walls of the calyces propels urine
through the renal pelvis and ureters to the bladder.
then into the major calyx. Peristalsis of the smooth muscle originating in pace-maker cells originating
in the walls of the calyces propels urine
through the renal pelvis and ureters to the bladder.
MAJOR CALYX
Two or three minor calyces converge to
form a major calyx. The major calyx, in the kidney, surrounds the apex of the renal pyramids. Urine formed in the kidney passes through a renal papilla at the apex into a minor calyx then into major calyx before passing through the renal pelvis into the ureter. Peristalsis of the smooth muscle originating in pace-maker cells originating
in the walls of the calyces propels urine
through the renal pelvis and ureters to the bladder.
form a major calyx. The major calyx, in the kidney, surrounds the apex of the renal pyramids. Urine formed in the kidney passes through a renal papilla at the apex into a minor calyx then into major calyx before passing through the renal pelvis into the ureter. Peristalsis of the smooth muscle originating in pace-maker cells originating
in the walls of the calyces propels urine
through the renal pelvis and ureters to the bladder.
RENAL PELVIS
The renal pelvis or pyelum is the funnel- like dilated proximal part of the ureter in the kidney. In humans, the renal pelvis is the point of
convergence of two or three major calyces. Each renal papilla is surrounded by a branch of the renal pelvis called a calyx. The major function of the renal pelvis is to
act as a funnel for urine flowing to the ureter. The renal pelvis is the location of several
kinds of kidney cancer. Its mucous membrane is covered with
transitional epithelium, and an underlying lamina propria of loose to dense connective tissue.
convergence of two or three major calyces. Each renal papilla is surrounded by a branch of the renal pelvis called a calyx. The major function of the renal pelvis is to
act as a funnel for urine flowing to the ureter. The renal pelvis is the location of several
kinds of kidney cancer. Its mucous membrane is covered with
transitional epithelium, and an underlying lamina propria of loose to dense connective tissue.
RENAL PYRAMID
Renal pyramids (or malpighian pyramids) are cone-shaped tissues of the kidney. The renal medulla is made up of 7 to 18 of these conical subdivisions (usually 7 in
humans). The broad base of each pyramid
faces the renal cortex, and its apex, or papilla, points internally. The pyramids appear striped because they are formed by
straight parallel segments of nephrons.
humans). The broad base of each pyramid
faces the renal cortex, and its apex, or papilla, points internally. The pyramids appear striped because they are formed by
straight parallel segments of nephrons.
Wednesday 5 October 2011
PEDICEL
The renal hilum (Latin hilum renale) or renal pedicle of the kidney is the recessed central fissure. The medial border of the kidney is concave in the center and convex toward either extremity; it is directed
forward and a little downward. Its central
part presents a deep longitudinal fissure,
bounded by prominent overhanging
anterior and posterior lips. This fissure is
named the hilum, and transmits the vessels, nerves, and ureter. From anterior to posterior, the renal vein exits, the renal
artery enters, and the renal pelvis exits the
kidney. Hilum's Order From anterior to posterior, the tubes
entering the hilum of kidney are renal vein, renal artery and ureter.
forward and a little downward. Its central
part presents a deep longitudinal fissure,
bounded by prominent overhanging
anterior and posterior lips. This fissure is
named the hilum, and transmits the vessels, nerves, and ureter. From anterior to posterior, the renal vein exits, the renal
artery enters, and the renal pelvis exits the
kidney. Hilum's Order From anterior to posterior, the tubes
entering the hilum of kidney are renal vein, renal artery and ureter.
BLOOD SUPPLY TO THE KIDNEY
1. Renal pyramid 2. Interlobular artery 3. Renal artery 4. Renal vein 5. Renal hilum 6. Renal pelvis 7. Ureter 8. Minor calyx 9. Renal capsule 10. Inferior renal capsule 11. Superior renal capsule 12. interlobular vein 13. cortical
nephron (in
cortex)
14. Minor calyx 15. Major calyx 16. Renal papilla 17. Renal column The renal circulation receives around 20% of the cardiac output. It branches from the abdominal aorta and returns blood to the ascending vena cava . It is the blood supply to the kidney, and contains many specialized blood vessels. Circulation The table below shows the path that blood
takes when it travels through the
glomerulus, traveling "down" the arteries,
and "up" the veins. However, this model is
greatly simplified for clarity and
symmetry. Some of the other paths and complications are described at the bottom
of the table. INTERLOBAR ARTERY & VEIN (not to be
confused with interlobular) are between 2
renal lobes, also known as the renal column
(cortex region between two pyramids). Arteries (down) Veins (up) Abdominal aorta Vena cava Renal artery (Note 1) Renal vein Segmental arteries (Note 2) - Lobar arteries - Interlobar artery Interlobar vein Arcuate arteries Arcuate vein Interlobular artery (Note 3) Interlobular vein Afferent arterioles Efferent arterioles (Note 4) Glomerulus Glomerulus Note 1: The renal artery also provides a branch to the inferior suprarenal artery to supply the adrenal gland. Note 2: Each renal artery partitions into
an anterior and posterior branch. The
anterior branch further divides into the
superior (apical), anterosuperior,
anteroinferior and inferior segmental
arteries. The posterior branch continues as the posterior segmental artery. Note 3: The interlobular artery also
supplies to the stellate veins. Note 4: The efferent arterioles don't
directly drain into the interlobular vein,
but rather they go to the peritubular capillaries first. The efferent arterioles of the juxtamedullary nephron drain into the vasa recta.
nephron (in
cortex)
14. Minor calyx 15. Major calyx 16. Renal papilla 17. Renal column The renal circulation receives around 20% of the cardiac output. It branches from the abdominal aorta and returns blood to the ascending vena cava . It is the blood supply to the kidney, and contains many specialized blood vessels. Circulation The table below shows the path that blood
takes when it travels through the
glomerulus, traveling "down" the arteries,
and "up" the veins. However, this model is
greatly simplified for clarity and
symmetry. Some of the other paths and complications are described at the bottom
of the table. INTERLOBAR ARTERY & VEIN (not to be
confused with interlobular) are between 2
renal lobes, also known as the renal column
(cortex region between two pyramids). Arteries (down) Veins (up) Abdominal aorta Vena cava Renal artery (Note 1) Renal vein Segmental arteries (Note 2) - Lobar arteries - Interlobar artery Interlobar vein Arcuate arteries Arcuate vein Interlobular artery (Note 3) Interlobular vein Afferent arterioles Efferent arterioles (Note 4) Glomerulus Glomerulus Note 1: The renal artery also provides a branch to the inferior suprarenal artery to supply the adrenal gland. Note 2: Each renal artery partitions into
an anterior and posterior branch. The
anterior branch further divides into the
superior (apical), anterosuperior,
anteroinferior and inferior segmental
arteries. The posterior branch continues as the posterior segmental artery. Note 3: The interlobular artery also
supplies to the stellate veins. Note 4: The efferent arterioles don't
directly drain into the interlobular vein,
but rather they go to the peritubular capillaries first. The efferent arterioles of the juxtamedullary nephron drain into the vasa recta.
RENAL PYRAMID
Renal pyramids (or malpighian pyramids) are cone-shaped tissues of the kidney. The renal medulla is made up of 7 to 18 of these conical subdivisions (usually 7 in
humans). The broad base of each pyramid
faces the renal cortex, and its apex, or papilla, points internally. The pyramids appear striped because they are formed by
straight parallel segments of nephrons.
humans). The broad base of each pyramid
faces the renal cortex, and its apex, or papilla, points internally. The pyramids appear striped because they are formed by
straight parallel segments of nephrons.
INTERLOBAR VEIN
The interlobar veins are veins of the renal circulation which drain the renal lobes.
RENAL VEIN
The renal veins are veins that drain the kidney. They connect the kidney to the inferior vena cava . It is usually singular to each kidney, except in the condition "multiple renal veins".[1] It also divides into 2 divisions upon
entering the kidney: the anterior branch which receives
blood from the anterior portion of the
kidney and, the posterior branch which receives
blood from the posterior portion. Often, each renal vein will have a branch
that receives blood from the ureter. Asymmetry Because the inferior vena cava is on the
right half of the body, the left renal vein is
generally the longer of the two. Because the inferior vena cava is not laterally symmetrical, the left renal vein often receives the following veins: [2] left inferior phrenic vein left suprarenal vein left gonadal vein (left testicular vein in males, left ovarian vein in females) left 2nd lumbar vein This is in contrast to the right side of the
body, where these veins drain directly into
the IVC. Pathology Diseases associated with the renal vein
include renal vein thrombosis (RVT) and nutcracker syndrome (renal vein entrapment syndrome).
entering the kidney: the anterior branch which receives
blood from the anterior portion of the
kidney and, the posterior branch which receives
blood from the posterior portion. Often, each renal vein will have a branch
that receives blood from the ureter. Asymmetry Because the inferior vena cava is on the
right half of the body, the left renal vein is
generally the longer of the two. Because the inferior vena cava is not laterally symmetrical, the left renal vein often receives the following veins: [2] left inferior phrenic vein left suprarenal vein left gonadal vein (left testicular vein in males, left ovarian vein in females) left 2nd lumbar vein This is in contrast to the right side of the
body, where these veins drain directly into
the IVC. Pathology Diseases associated with the renal vein
include renal vein thrombosis (RVT) and nutcracker syndrome (renal vein entrapment syndrome).
RENAL ARTERY
The renal arteries normally arise off the side of the abdominal aorta, immediately below the superior mesenteric artery, and supply the kidneys with blood. Each is directed across the crus of the diaphragm, so as to form nearly a right angle with the
aorta. The renal arteries carry a large portion of
total blood flow to the kidneys. Up to a
third of total cardiac output can pass
through the renal arteries to be filtered by
the kidneys. The arterial supply of the kidneys is
variable and there may be one or more
renal arteries supplying each kidney. It is
located above the renal vein.
Supernumerary renal arteries(two or more
arteries to a single kidney) are the most common renovascular anomaly, occurrence
ranging from 25% to 40% of kidneys. It has a radius of approximately 0.25 cm,[1] 0.26 cm at the root.[2] The measured mean diameter can differ depending on the
imaging method used. For example, the
diameter was found to be 5.04 ± 0.74 mm
using ultrasound, but 5.68 ± 1.19 mm using angiography.[3] Asymmetries before reaching kidney Due to the position of the aorta, the inferior vena cava and the kidneys in the body, the right renal artery is normally
longer than the left renal artery. The right passes behind the inferior vena cava , the right renal vein, the head of the pancreas, and the descending part of the duodenum. The left is somewhat higher than the
right; it lies behind the left renal vein,
the body of the pancreas and the splenic vein, and is crossed by the inferior mesenteric vein. At kidney Before reaching the hilus of the kidney, each artery divides into four or five
branches; the greater number of these
(anterior branches) lie between the renal
vein and ureter, the vein being in front, the ureter behind, but one or more branches
(posterior branches) are usually situated
behind the ureter. Each vessel gives off some small inferior suprarenal branches to the suprarenal gland, the ureter, and the surrounding cellular tissue and muscles. One or two accessory renal arteries are
frequently found, especially on the left side
since they usually arise from the aorta, and
may come off above (more common) or
below the main artery. Instead of entering
the kidney at the hilus, they usually pierce the upper or lower part of the organ. Diseases of the renal arteries Renal artery stenosis, or narrowing of one or both renal arteries will lead to
hypertension as the affected kidneys
release renin to increase blood pressure to preserve perfusion to the kidneys. RAS is
typically diagnosed with duplex
ultrasonography of the renal arteries. It is
treated with the use of balloon angioplasty
and stents, if necessary. Atherosclerosis can also affect the renal arteries and can lead to poor perfusion of
the kidneys leading to reduced kidney
function and, possibly, renal failure.
aorta. The renal arteries carry a large portion of
total blood flow to the kidneys. Up to a
third of total cardiac output can pass
through the renal arteries to be filtered by
the kidneys. The arterial supply of the kidneys is
variable and there may be one or more
renal arteries supplying each kidney. It is
located above the renal vein.
Supernumerary renal arteries(two or more
arteries to a single kidney) are the most common renovascular anomaly, occurrence
ranging from 25% to 40% of kidneys. It has a radius of approximately 0.25 cm,[1] 0.26 cm at the root.[2] The measured mean diameter can differ depending on the
imaging method used. For example, the
diameter was found to be 5.04 ± 0.74 mm
using ultrasound, but 5.68 ± 1.19 mm using angiography.[3] Asymmetries before reaching kidney Due to the position of the aorta, the inferior vena cava and the kidneys in the body, the right renal artery is normally
longer than the left renal artery. The right passes behind the inferior vena cava , the right renal vein, the head of the pancreas, and the descending part of the duodenum. The left is somewhat higher than the
right; it lies behind the left renal vein,
the body of the pancreas and the splenic vein, and is crossed by the inferior mesenteric vein. At kidney Before reaching the hilus of the kidney, each artery divides into four or five
branches; the greater number of these
(anterior branches) lie between the renal
vein and ureter, the vein being in front, the ureter behind, but one or more branches
(posterior branches) are usually situated
behind the ureter. Each vessel gives off some small inferior suprarenal branches to the suprarenal gland, the ureter, and the surrounding cellular tissue and muscles. One or two accessory renal arteries are
frequently found, especially on the left side
since they usually arise from the aorta, and
may come off above (more common) or
below the main artery. Instead of entering
the kidney at the hilus, they usually pierce the upper or lower part of the organ. Diseases of the renal arteries Renal artery stenosis, or narrowing of one or both renal arteries will lead to
hypertension as the affected kidneys
release renin to increase blood pressure to preserve perfusion to the kidneys. RAS is
typically diagnosed with duplex
ultrasonography of the renal arteries. It is
treated with the use of balloon angioplasty
and stents, if necessary. Atherosclerosis can also affect the renal arteries and can lead to poor perfusion of
the kidneys leading to reduced kidney
function and, possibly, renal failure.
SEGMENTAL ARTERY
The segmental arteries are branches of the renal arteries. There are five named segmental arteries: [1][2] superior inferior anterior anterior superior anterior inferior posterior
interlobar artery
The interlobar arteries are vessels of the renal circulation which supply the renal lobes.
RENAL CORTEX
The renal cortex is the outer portion of the kidney between the renal capsule and the renal medulla. In the adult, it forms a continuous smooth outer zone with a
number of projections (cortical columns) that extend down between the pyramids. It contains the renal corpuscles and the renal tubules except for parts of the loop of Henle which descend into the renal medulla. It also contains blood vessels and cortical collecting ducts. The renal cortex is the part of the kidney where ultrafiltration occurs. Erythropoietin is produced in the renal cortex.
number of projections (cortical columns) that extend down between the pyramids. It contains the renal corpuscles and the renal tubules except for parts of the loop of Henle which descend into the renal medulla. It also contains blood vessels and cortical collecting ducts. The renal cortex is the part of the kidney where ultrafiltration occurs. Erythropoietin is produced in the renal cortex.
INTERLOBULAR ARTERY
The first set of renal bloodvessels, the interlobular arteries (or cortical radiate arteries, or cortical radial arteries), are given off at right angles from the side of
the arcuate arteries looking toward the cortical substance, and pass directly
outward between the medullary rays to reach the fibrous tunic, where they end in
the capillary network of this part. These vessels do not anastomose with
each other, but form what are called end-
arteries. In their outward course they give off
lateral branches; these are the afferent vessels for the renal corpuscles; they enter the capsule, and end in the glomerulus. From each tuft the corresponding efferent
vessel arises, and, having made its egress
from the capsule near to the point where
the afferent vessel enters, breaks up into a
number of branches, which form a dense
plexus within Bowman's capsule.
the arcuate arteries looking toward the cortical substance, and pass directly
outward between the medullary rays to reach the fibrous tunic, where they end in
the capillary network of this part. These vessels do not anastomose with
each other, but form what are called end-
arteries. In their outward course they give off
lateral branches; these are the afferent vessels for the renal corpuscles; they enter the capsule, and end in the glomerulus. From each tuft the corresponding efferent
vessel arises, and, having made its egress
from the capsule near to the point where
the afferent vessel enters, breaks up into a
number of branches, which form a dense
plexus within Bowman's capsule.
PERITUBULAR CAPILLARIES
In the renal system, peritubular capillaries are tiny blood vessels that travel alongside nephrons allowing reabsorption and secretion between blood and the inner lumen of the nephron. Ions and minerals that need to be saved in
the body are reabsorbed into the
peritubular capillaries through active transport, secondary active transport, or transcytosis. The ions that need to be excreted as waste are secreted from the capillaries into the
nephron to be sent towards the bladder and out of the body. The majority of exchange through the
peritubular capillaries occurs because of
chemical gradients, osmosis and Na+ pumps.
the body are reabsorbed into the
peritubular capillaries through active transport, secondary active transport, or transcytosis. The ions that need to be excreted as waste are secreted from the capillaries into the
nephron to be sent towards the bladder and out of the body. The majority of exchange through the
peritubular capillaries occurs because of
chemical gradients, osmosis and Na+ pumps.
VASA RECTA JUXTAMEDULLARY NEPHRON
In the blood supply of the kidney , the vasa recta renis (or straight arteries of kidney, or straight arterioles of kidney) form a series of straight capillaries (recta is from
the Latin for "straight") in the medulla. They lie parallel to the loop of Henle. These vessels branch off the efferent arterioles of juxtamedullary nephrons (those nephrons closest to the medulla), enter the medulla, and surround the loop of Henle. Histology On a slide, vasa recta can be distinguished
from the tubules of the loop of Henle by the presence of blood.[1] Function Each of the vasa recta has a hairpin turn in
the medulla and carries blood at a very
slow rate, two factors crucial in the
maintenance of countercurrent exchange that prevent washout of the concentration
gradients established in the renal medulla. [2] The maintenance of this concentration
gradient is one of the components
responsible for the kidney's ability to
produce concentrated urine. On the descending portion of the vasa
recta, NaCl and urea are reabsorbed into
the blood, while water is secreted. On the
ascending portion of the vasa recta, NaCl
and urea are secreted into the interstitium,
while water is reabsorbed. Nomenclature According to Terminologia Anatomica , the term "vasa recta renis" is an alternate
name for "arteriolae rectae renis", and a
separate term, venulae rectae renis, is used to identify the venous portion. However, other sources consider "vasa
recta renis" to refer to both the arterial and venous portions.[3] The "renis" is often omitted, but there do
exist two other structures with the same
name: vasa recta (intestines) (in the ileum and jejunum)[4] the straight portion of the seminiferous tubule, though this is usually called the tubuli recti. Pathology The slow blood flow in vasa recta makes
them a likely location of thrombosis in hypercoagulable states, or tissue loss[5] due to erythrocyte sickling in sickle cell disease. Ischemia that results may lead to renal papillary necrosis.
the Latin for "straight") in the medulla. They lie parallel to the loop of Henle. These vessels branch off the efferent arterioles of juxtamedullary nephrons (those nephrons closest to the medulla), enter the medulla, and surround the loop of Henle. Histology On a slide, vasa recta can be distinguished
from the tubules of the loop of Henle by the presence of blood.[1] Function Each of the vasa recta has a hairpin turn in
the medulla and carries blood at a very
slow rate, two factors crucial in the
maintenance of countercurrent exchange that prevent washout of the concentration
gradients established in the renal medulla. [2] The maintenance of this concentration
gradient is one of the components
responsible for the kidney's ability to
produce concentrated urine. On the descending portion of the vasa
recta, NaCl and urea are reabsorbed into
the blood, while water is secreted. On the
ascending portion of the vasa recta, NaCl
and urea are secreted into the interstitium,
while water is reabsorbed. Nomenclature According to Terminologia Anatomica , the term "vasa recta renis" is an alternate
name for "arteriolae rectae renis", and a
separate term, venulae rectae renis, is used to identify the venous portion. However, other sources consider "vasa
recta renis" to refer to both the arterial and venous portions.[3] The "renis" is often omitted, but there do
exist two other structures with the same
name: vasa recta (intestines) (in the ileum and jejunum)[4] the straight portion of the seminiferous tubule, though this is usually called the tubuli recti. Pathology The slow blood flow in vasa recta makes
them a likely location of thrombosis in hypercoagulable states, or tissue loss[5] due to erythrocyte sickling in sickle cell disease. Ischemia that results may lead to renal papillary necrosis.
INTERLOBULAR VEIN
The venae stellatae join to form the interlobular veins, which pass inward between the rays, receive branches from the plexuses around the convoluted tubules, and, having arrived at the bases of
the renal pyramids, join with the venae rectae.
the renal pyramids, join with the venae rectae.
JUXTAGLOMERULAR APPARATUS
The juxtaglomerular apparatus is a microscopic structure in the kidney, which regulates the function of each nephron. The juxtaglomerular apparatus is named for its
proximity to the glomerulus: it is found between the vascular pole of the renal corpuscle and the returning distal convoluted tubule of the same nephron. This location is critical to its function in
regulating renal blood flow and glomerular filtration rate. The three cellular components of the apparatus are the macula densa, extraglomerular mesangial cells, and juxtaglomerular cells (juxtaglomerular cells are not granular cells
but are granulated as they release Renin). Cells of the Juxtaglomerular Apparatus There are 3 different types of cells in the
Juxtaglomerular Apparatus: Granular Cells,
Macula Densa Cells, and Mesangial Cells. Granular Cells Granular cells are modified pericytes of
glomerular arterioles. They are also known
as Juxtaglomerular cells.. The Juxtaglomerular cells secrete renin in response to: Beta1 adrenergic stimulation Decrease in renal perfusion pressure
(detected directly by the granular cells) Decrease in NaCl absorption in the
Macula Densa (often due to a decrease in glomerular filtration rate, or GFR, causing slower filtrate movement
through the proximal tubule and thus
more time for reabsorption. This results
in a lower NaCl concentration by the
time the filtrate reaches the Macula
Densa). Macula Densa Cells Macula densa cells are columnar epithelium
thickening of the distal tubule. The macula
densa senses sodium chloride concentration in the distal tubule of the
kidney and secretes a locally active
(paracrine) vasopressor which acts on the adjacent afferent arteriole to decrease glomerular filtration rate (GFR), as part of the tubuloglomerular feedback loop. Specifically, excessive filtration at the
glomerulus or inadequate sodium uptake in
the proximal tubule / thick ascending loop
of Henle brings fluid to the distal
convoluted tubule that has an abnormally
high concentration of sodium. Na/Cl cotransporters move sodium into the cells of the macula densa. The macula densa
cells do not have enough basolateral Na/K ATPases to excrete this added sodium, so the cell's osmolarity increases. Water flows into the cell to bring the osmolarity back
down, causing the cell to swell. When the
cell swells, a stretch-activated non-
selective anion channel is opened on the basolateral surface. ATP escapes through this channel and is subsequently converted
to adenosine. Adenosine vasoconstricts the afferent arteriole via A1 receptors and
vasodilates (to a lesser degree) efferent
arterioles via A2 receptors which
decreases GFR. Also, adenosine inhibits
renin release in JG cells via A2 receptors on
JG cells using Gi pathway. Also, when macula densa cells detect higher
concentrations of Na and Cl they inhibit
Nitric Oxide Synthetase (decreasing renin
release) with an unknown pathway. A decrease in GFR means less solute in the
tubular lumen. As the filtrate reaches the
macula densa, less NaCl is re-absorbed. The
macula densa cells detect lower
concentrations in Na and Cl and upregulate
Nitric Oxide Synthetase (NOS). NOS creates NO which catalyses the formation of
prostaglandins. These prostaglandins
diffuse to the granular cells and activate a
prostaglandin specific Gs receptor. This
receptor activates adenylate cyclase which
increases levels of cAMP. cAMP augments renin release. Prostaglandins and NO also
makes a vasadilator effect on afferent
arteriol but this doesn't happen on efferent
arteriol due to renin release. Mesangial cells Mesangial cells are structural cells in the glomerulus that under normal conditions
serve as anchors for the glomerular
capillaries. The mesangial cells within the
glomerulus communicate with mesangial
cells outside the glomerulus
(extraglomerular mesangial cells), and it is the latter cells that form part of the
juxtaglomerular apparatus. These cells
form a syncytium and are connected with glomerular mesangial cells via gap junctions. The function of the extraglomerular
mesangial cells remains somewhat
mysterious. They contain actin and myosin, allowing them to contract when stimulated
by renal sympathetic nerves , which may provide a way for the sympathetic nervous
system to modulate the actions of the
juxtaglomerular apparatus. The latest
thinking is that in times of great
sympathetic discharge [i.e. during periods
when the blood pressure is low, e.g. from blood loss ], mesangial contraction reduces
the surface area of the glomerulae, thus
reducing glomerular filtration and saving
excess fluid from being lost into the urine.
In addition, extraglomerular mesangial
cells are strategically positioned between the macula densa and the afferent
arteriole, and may mediate signalling between these two structures.
proximity to the glomerulus: it is found between the vascular pole of the renal corpuscle and the returning distal convoluted tubule of the same nephron. This location is critical to its function in
regulating renal blood flow and glomerular filtration rate. The three cellular components of the apparatus are the macula densa, extraglomerular mesangial cells, and juxtaglomerular cells (juxtaglomerular cells are not granular cells
but are granulated as they release Renin). Cells of the Juxtaglomerular Apparatus There are 3 different types of cells in the
Juxtaglomerular Apparatus: Granular Cells,
Macula Densa Cells, and Mesangial Cells. Granular Cells Granular cells are modified pericytes of
glomerular arterioles. They are also known
as Juxtaglomerular cells.. The Juxtaglomerular cells secrete renin in response to: Beta1 adrenergic stimulation Decrease in renal perfusion pressure
(detected directly by the granular cells) Decrease in NaCl absorption in the
Macula Densa (often due to a decrease in glomerular filtration rate, or GFR, causing slower filtrate movement
through the proximal tubule and thus
more time for reabsorption. This results
in a lower NaCl concentration by the
time the filtrate reaches the Macula
Densa). Macula Densa Cells Macula densa cells are columnar epithelium
thickening of the distal tubule. The macula
densa senses sodium chloride concentration in the distal tubule of the
kidney and secretes a locally active
(paracrine) vasopressor which acts on the adjacent afferent arteriole to decrease glomerular filtration rate (GFR), as part of the tubuloglomerular feedback loop. Specifically, excessive filtration at the
glomerulus or inadequate sodium uptake in
the proximal tubule / thick ascending loop
of Henle brings fluid to the distal
convoluted tubule that has an abnormally
high concentration of sodium. Na/Cl cotransporters move sodium into the cells of the macula densa. The macula densa
cells do not have enough basolateral Na/K ATPases to excrete this added sodium, so the cell's osmolarity increases. Water flows into the cell to bring the osmolarity back
down, causing the cell to swell. When the
cell swells, a stretch-activated non-
selective anion channel is opened on the basolateral surface. ATP escapes through this channel and is subsequently converted
to adenosine. Adenosine vasoconstricts the afferent arteriole via A1 receptors and
vasodilates (to a lesser degree) efferent
arterioles via A2 receptors which
decreases GFR. Also, adenosine inhibits
renin release in JG cells via A2 receptors on
JG cells using Gi pathway. Also, when macula densa cells detect higher
concentrations of Na and Cl they inhibit
Nitric Oxide Synthetase (decreasing renin
release) with an unknown pathway. A decrease in GFR means less solute in the
tubular lumen. As the filtrate reaches the
macula densa, less NaCl is re-absorbed. The
macula densa cells detect lower
concentrations in Na and Cl and upregulate
Nitric Oxide Synthetase (NOS). NOS creates NO which catalyses the formation of
prostaglandins. These prostaglandins
diffuse to the granular cells and activate a
prostaglandin specific Gs receptor. This
receptor activates adenylate cyclase which
increases levels of cAMP. cAMP augments renin release. Prostaglandins and NO also
makes a vasadilator effect on afferent
arteriol but this doesn't happen on efferent
arteriol due to renin release. Mesangial cells Mesangial cells are structural cells in the glomerulus that under normal conditions
serve as anchors for the glomerular
capillaries. The mesangial cells within the
glomerulus communicate with mesangial
cells outside the glomerulus
(extraglomerular mesangial cells), and it is the latter cells that form part of the
juxtaglomerular apparatus. These cells
form a syncytium and are connected with glomerular mesangial cells via gap junctions. The function of the extraglomerular
mesangial cells remains somewhat
mysterious. They contain actin and myosin, allowing them to contract when stimulated
by renal sympathetic nerves , which may provide a way for the sympathetic nervous
system to modulate the actions of the
juxtaglomerular apparatus. The latest
thinking is that in times of great
sympathetic discharge [i.e. during periods
when the blood pressure is low, e.g. from blood loss ], mesangial contraction reduces
the surface area of the glomerulae, thus
reducing glomerular filtration and saving
excess fluid from being lost into the urine.
In addition, extraglomerular mesangial
cells are strategically positioned between the macula densa and the afferent
arteriole, and may mediate signalling between these two structures.
NEPHRON
Nephron (from Greek νεφρός - nephros, meaning "kidney") is the basic structural
and functional unit of the kidney. Its chief function is to regulate the concentration of water and soluble substances like sodium salts by filtering the blood, reabsorbing what is needed and excreting the rest as urine. A nephron eliminates wastes from the body, regulates blood volume and blood pressure, controls levels of electrolytes and metabolites, and regulates blood pH. Its functions are vital to life and are regulated by the endocrine system by hormones such as antidiuretic hormone, aldosterone, and parathyroid hormone.[1] In humans, a normal kidney contains 800,000 to 1.5 million nephrons.[2] Types of nephrons Two general classes of nephrons are
cortical nephrons and juxtamedullary nephrons, both of which are classified according to the length of their associated Loop of Henle and location of their renal corpuscle. All nephrons have their renal corpuscles in the cortex. Cortical nephrons
have their Loop of Henle in the renal medulla near its junction with the renal cortex, while the Loop of Henle of juxtamedullary nephrons is located deep in
the renal medulla; they are called
juxtamedullary because their renal
corpuscle is located near the medulla (but
still in the cortex). The nomenclature for
cortical nephrons varies, with some sources distinguishing between superficial
cortical nephrons and midcortical nephrons,
depending on where their corpuscle is located within the cortex.[3] The majority of nephrons are cortical.
Cortical nephrons have a shorter loop of Henle compared to juxtamedullary nephrons. The longer loop of Henle in
juxtamedullary nephrons create a
hyperosmolar gradient that allows for the creation of concentrated urine.[4] Anatomy Each nephron is composed of an initial
filtering component (the "renal corpuscle") and a tubule specialized for reabsorption
and secretion (the "renal tubule"). The
renal corpuscle filters out large solutes
from the blood, delivering water and small
solutes to the renal tubule for modification.[citation needed] Renal corpuscle Composed of a glomerulus and the Bowman's capsule, the renal corpuscle (or Malpighian corpuscle) is the beginning of the nephron. It is the nephron's initial filtering component.[citation needed] The glomerulus is a capillary tuft that receives its blood supply from an afferent arteriole of the renal circulation. The glomerular blood pressure provides the
driving force for water and solutes to be
filtered out of the blood and into the space
made by Bowman's capsule. The remainder of the blood (only approximately 1/5 of all
plasma passing through the kidney is
filtered through the glomerular wall into
the Bowman's capsule) passes into the
efferent arteriole.The diameter of efferent
arteriole is comparatively less than that of afferent arteriole. It then moves into the
vasa recta, which are only found in
juxtamedullary nephrons and not cortical
nephrons. The vasa recta are collecting
capillaries intertwined with the
convoluted tubules through the interstitial space, in which the reabsorbed substances
will also enter. This then combines with
efferent venules from other nephrons into
the renal vein, and rejoins the main bloodstream.[citation needed] The Bowman's capsule, also called the glomerular capsule, surrounds the
glomerulus. It is composed of a visceral
inner layer formed by specialized cells
called podocytes, and a parietal outer layer composed of a single layer of flat cells
called simple squamous epithelium. Fluids from blood in the glomerulus are filtered
through the visceral layer of podocytes,
and the resulting glomerular filtrate is further processed along the nephron to form urine.[citation needed] Renal tubule Renal tubule Latin tubulus renalis Gray's subject #253 1223 The renal tubule is the portion of the
nephron containing the tubular fluid filtered through the glomerulus.[5] After passing through the renal tubule, the
filtrate continues to the collecting duct system, which is not part of the nephron.[citation needed] The components of the renal tubule are: Proximal convoluted tubule (lies in cortex and lined by simple cuboidal
epithelium with brushed borders which
help to increase the area of absorption
greatly.) Loop of Henle (hair-pin like i.e. U-shaped and lies in medulla) Descending limb of loop of Henle Ascending limb of loop of Henle The ascending limb of loop of
Henle is divided into 2 segments:
Lower end of ascending limb is
very thin and is lined by simple
squamous epithelium. The distal
portion of ascending limb is thick and is lined by simple cuboidal
epithelium. Thin ascending limb of loop of
Henle Thick ascending limb of loop of
Henle (enters cortex and becomes DCT-distal convoluted tubule.) Distal convoluted tubule Functions The nephron carries out nearly all of the
kidney's functions. Most of these functions
concern the reabsorption and secretion of various solutes such as ions (e.g., sodium), carbohydrates (e.g., glucose), and amino acids (e.g., glutamate). Properties of the cells that line the nephron change
dramatically along its length; consequently,
each segment of the nephron has highly specialized functions.[citation needed] The proximal tubule as a part of the
nephron can be divided into an initial
convoluted portion and a following straight (descending) portion.[6] Fluid in the filtrate entering the proximal convoluted tubule is
reabsorbed into the peritubular capillaries,
including approximately two-thirds of the
filtered salt and water and all filtered organic solutes (primarily glucose and amino acids).[citation needed] The loop of Henle, also called the nephron loop, is a U-shaped tube that extends from
the proximal tubule. It consists of a
descending limb and ascending limb. It
begins in the cortex, receiving filtrate from
the proximal convoluted tubule, extends
into the medulla as the descending limb, and then returns to the cortex as the
ascending limb to empty into the distal
convoluted tubule. The primary role of the
loop of Henle is to concentrate the salt in
the interstitium, the tissue surrounding the loop.[citation needed] Considerable differences distinguish the
descending and ascending limbs of the loop
of Henle. The descending limb is permeable to water and noticeably less impermeable
to salt, and thus only indirectly contributes
to the concentration of the interstitium. As
the filtrate descends deeper into the hypertonic interstitium of the renal medulla, water flows freely out of the
descending limb by osmosis until the tonicity of the filtrate and interstitium
equilibrate. Longer descending limbs allow
more time for water to flow out of the
filtrate, so longer limbs make the filtrate
more hypertonic than shorter limbs.[citation needed] Unlike the descending limb, the ascending limb of Henle's loop[disambiguation needed] is impermeable to water, a critical feature of
the countercurrent exchange mechanism employed by the loop. The ascending limb
actively pumps sodium out of the filtrate,
generating the hypertonic interstitium that
drives countercurrent exchange. In passing
through the ascending limb, the filtrate
grows hypotonic since it has lost much of its sodium content. This hypotonic filtrate
is passed to the distal convoluted tubule in the renal cortex.[citation needed] The distal convoluted tubule has a different structure and function to that of the
proximal convoluted tubule. Cells lining the
tubule have numerous mitochondria to produce enough energy (ATP) for active transport to take place. Much of the ion transport taking place in the distal
convoluted tubule is regulated by the endocrine system. In the presence of parathyroid hormone, the distal convoluted tubule reabsorbs more calcium and
excretes more phosphate. When aldosterone is present, more sodium is reabsorbed and more potassium excreted. Atrial natriuretic peptide causes the distal convoluted tubule to excrete more sodium.
In addition, the tubule also secretes hydrogen and ammonium to regulate pH.[citation needed] After traveling the length of the distal
convoluted tubule, only about 1% of water
remains, and the remaining salt content is negligible.[citation needed] Collecting duct system Main article: Collecting duct system Each distal convoluted tubule delivers its
filtrate to a system of collecting ducts, the first segment of which is the collecting tubule. The collecting duct system begins in the renal cortex and extends deep into the
medulla. As the urine travels down the
collecting duct system, it passes by the
medullary interstitium which has a high
sodium concentration as a result of the
loop of Henle's countercurrent multiplier system.[citation needed] Though the collecting duct is normally
impermeable to water, it becomes
permeable in the presence of antidiuretic hormone (ADH). ADH affects the function of aquaporins, resulting in the reabsorption of water molecules as it passes through the
collecting duct. Aquaporins are membrane
proteins that selectively conduct water
molecules while preventing the passage of
ions and other solutes. As much as three-
quarters of the water from urine can be reabsorbed as it leaves the collecting duct
by osmosis. Thus the levels of ADH
determine whether urine will be
concentrated or diluted. An increase in ADH
is an indication of dehydration, while water sufficiency results in low ADH allowing for diluted urine.[citation needed] Lower portions of the collecting organ are
also permeable to urea, allowing some of it to enter the medulla of the kidney, thus maintaining its high concentration (which
is very important for the nephron).[citation needed] Urine leaves the medullary collecting ducts
through the renal papillae, emptying into the renal calyces, the renal pelvis, and finally into the urinary bladder via the ureter.[citation needed] Because it has a different origin during the development of the urinary and
reproductive organs than the rest of the nephron, the collecting duct is sometimes
not considered a part of the nephron.
Instead of originating from the
metanephrogenic blastema, the collecting
duct originates from the ureteric bud.[citation needed] Juxtaglomerular apparatus Main article: Juxtaglomerular apparatus The juxtaglomerular apparatus is a specialized region of the nephron
responsible for production and secretion of
the hormone renin, involved in the renin- angiotensin system. This apparatus occurs near the site of contact between the thick
ascending limb and the afferent arteriole.
It contains three components: the macula densa, juxtaglomerular cells, and extraglomerular mesangial cells.[citation needed] Clinical relevance Because of its importance in body fluid
regulation, the nephron is a common target
of drugs that treat high blood pressure and edema. These drugs, called diuretics, inhibit the ability of the nephron to retain
water, thereby increasing the amount of urine produced.
and functional unit of the kidney. Its chief function is to regulate the concentration of water and soluble substances like sodium salts by filtering the blood, reabsorbing what is needed and excreting the rest as urine. A nephron eliminates wastes from the body, regulates blood volume and blood pressure, controls levels of electrolytes and metabolites, and regulates blood pH. Its functions are vital to life and are regulated by the endocrine system by hormones such as antidiuretic hormone, aldosterone, and parathyroid hormone.[1] In humans, a normal kidney contains 800,000 to 1.5 million nephrons.[2] Types of nephrons Two general classes of nephrons are
cortical nephrons and juxtamedullary nephrons, both of which are classified according to the length of their associated Loop of Henle and location of their renal corpuscle. All nephrons have their renal corpuscles in the cortex. Cortical nephrons
have their Loop of Henle in the renal medulla near its junction with the renal cortex, while the Loop of Henle of juxtamedullary nephrons is located deep in
the renal medulla; they are called
juxtamedullary because their renal
corpuscle is located near the medulla (but
still in the cortex). The nomenclature for
cortical nephrons varies, with some sources distinguishing between superficial
cortical nephrons and midcortical nephrons,
depending on where their corpuscle is located within the cortex.[3] The majority of nephrons are cortical.
Cortical nephrons have a shorter loop of Henle compared to juxtamedullary nephrons. The longer loop of Henle in
juxtamedullary nephrons create a
hyperosmolar gradient that allows for the creation of concentrated urine.[4] Anatomy Each nephron is composed of an initial
filtering component (the "renal corpuscle") and a tubule specialized for reabsorption
and secretion (the "renal tubule"). The
renal corpuscle filters out large solutes
from the blood, delivering water and small
solutes to the renal tubule for modification.[citation needed] Renal corpuscle Composed of a glomerulus and the Bowman's capsule, the renal corpuscle (or Malpighian corpuscle) is the beginning of the nephron. It is the nephron's initial filtering component.[citation needed] The glomerulus is a capillary tuft that receives its blood supply from an afferent arteriole of the renal circulation. The glomerular blood pressure provides the
driving force for water and solutes to be
filtered out of the blood and into the space
made by Bowman's capsule. The remainder of the blood (only approximately 1/5 of all
plasma passing through the kidney is
filtered through the glomerular wall into
the Bowman's capsule) passes into the
efferent arteriole.The diameter of efferent
arteriole is comparatively less than that of afferent arteriole. It then moves into the
vasa recta, which are only found in
juxtamedullary nephrons and not cortical
nephrons. The vasa recta are collecting
capillaries intertwined with the
convoluted tubules through the interstitial space, in which the reabsorbed substances
will also enter. This then combines with
efferent venules from other nephrons into
the renal vein, and rejoins the main bloodstream.[citation needed] The Bowman's capsule, also called the glomerular capsule, surrounds the
glomerulus. It is composed of a visceral
inner layer formed by specialized cells
called podocytes, and a parietal outer layer composed of a single layer of flat cells
called simple squamous epithelium. Fluids from blood in the glomerulus are filtered
through the visceral layer of podocytes,
and the resulting glomerular filtrate is further processed along the nephron to form urine.[citation needed] Renal tubule Renal tubule Latin tubulus renalis Gray's subject #253 1223 The renal tubule is the portion of the
nephron containing the tubular fluid filtered through the glomerulus.[5] After passing through the renal tubule, the
filtrate continues to the collecting duct system, which is not part of the nephron.[citation needed] The components of the renal tubule are: Proximal convoluted tubule (lies in cortex and lined by simple cuboidal
epithelium with brushed borders which
help to increase the area of absorption
greatly.) Loop of Henle (hair-pin like i.e. U-shaped and lies in medulla) Descending limb of loop of Henle Ascending limb of loop of Henle The ascending limb of loop of
Henle is divided into 2 segments:
Lower end of ascending limb is
very thin and is lined by simple
squamous epithelium. The distal
portion of ascending limb is thick and is lined by simple cuboidal
epithelium. Thin ascending limb of loop of
Henle Thick ascending limb of loop of
Henle (enters cortex and becomes DCT-distal convoluted tubule.) Distal convoluted tubule Functions The nephron carries out nearly all of the
kidney's functions. Most of these functions
concern the reabsorption and secretion of various solutes such as ions (e.g., sodium), carbohydrates (e.g., glucose), and amino acids (e.g., glutamate). Properties of the cells that line the nephron change
dramatically along its length; consequently,
each segment of the nephron has highly specialized functions.[citation needed] The proximal tubule as a part of the
nephron can be divided into an initial
convoluted portion and a following straight (descending) portion.[6] Fluid in the filtrate entering the proximal convoluted tubule is
reabsorbed into the peritubular capillaries,
including approximately two-thirds of the
filtered salt and water and all filtered organic solutes (primarily glucose and amino acids).[citation needed] The loop of Henle, also called the nephron loop, is a U-shaped tube that extends from
the proximal tubule. It consists of a
descending limb and ascending limb. It
begins in the cortex, receiving filtrate from
the proximal convoluted tubule, extends
into the medulla as the descending limb, and then returns to the cortex as the
ascending limb to empty into the distal
convoluted tubule. The primary role of the
loop of Henle is to concentrate the salt in
the interstitium, the tissue surrounding the loop.[citation needed] Considerable differences distinguish the
descending and ascending limbs of the loop
of Henle. The descending limb is permeable to water and noticeably less impermeable
to salt, and thus only indirectly contributes
to the concentration of the interstitium. As
the filtrate descends deeper into the hypertonic interstitium of the renal medulla, water flows freely out of the
descending limb by osmosis until the tonicity of the filtrate and interstitium
equilibrate. Longer descending limbs allow
more time for water to flow out of the
filtrate, so longer limbs make the filtrate
more hypertonic than shorter limbs.[citation needed] Unlike the descending limb, the ascending limb of Henle's loop[disambiguation needed] is impermeable to water, a critical feature of
the countercurrent exchange mechanism employed by the loop. The ascending limb
actively pumps sodium out of the filtrate,
generating the hypertonic interstitium that
drives countercurrent exchange. In passing
through the ascending limb, the filtrate
grows hypotonic since it has lost much of its sodium content. This hypotonic filtrate
is passed to the distal convoluted tubule in the renal cortex.[citation needed] The distal convoluted tubule has a different structure and function to that of the
proximal convoluted tubule. Cells lining the
tubule have numerous mitochondria to produce enough energy (ATP) for active transport to take place. Much of the ion transport taking place in the distal
convoluted tubule is regulated by the endocrine system. In the presence of parathyroid hormone, the distal convoluted tubule reabsorbs more calcium and
excretes more phosphate. When aldosterone is present, more sodium is reabsorbed and more potassium excreted. Atrial natriuretic peptide causes the distal convoluted tubule to excrete more sodium.
In addition, the tubule also secretes hydrogen and ammonium to regulate pH.[citation needed] After traveling the length of the distal
convoluted tubule, only about 1% of water
remains, and the remaining salt content is negligible.[citation needed] Collecting duct system Main article: Collecting duct system Each distal convoluted tubule delivers its
filtrate to a system of collecting ducts, the first segment of which is the collecting tubule. The collecting duct system begins in the renal cortex and extends deep into the
medulla. As the urine travels down the
collecting duct system, it passes by the
medullary interstitium which has a high
sodium concentration as a result of the
loop of Henle's countercurrent multiplier system.[citation needed] Though the collecting duct is normally
impermeable to water, it becomes
permeable in the presence of antidiuretic hormone (ADH). ADH affects the function of aquaporins, resulting in the reabsorption of water molecules as it passes through the
collecting duct. Aquaporins are membrane
proteins that selectively conduct water
molecules while preventing the passage of
ions and other solutes. As much as three-
quarters of the water from urine can be reabsorbed as it leaves the collecting duct
by osmosis. Thus the levels of ADH
determine whether urine will be
concentrated or diluted. An increase in ADH
is an indication of dehydration, while water sufficiency results in low ADH allowing for diluted urine.[citation needed] Lower portions of the collecting organ are
also permeable to urea, allowing some of it to enter the medulla of the kidney, thus maintaining its high concentration (which
is very important for the nephron).[citation needed] Urine leaves the medullary collecting ducts
through the renal papillae, emptying into the renal calyces, the renal pelvis, and finally into the urinary bladder via the ureter.[citation needed] Because it has a different origin during the development of the urinary and
reproductive organs than the rest of the nephron, the collecting duct is sometimes
not considered a part of the nephron.
Instead of originating from the
metanephrogenic blastema, the collecting
duct originates from the ureteric bud.[citation needed] Juxtaglomerular apparatus Main article: Juxtaglomerular apparatus The juxtaglomerular apparatus is a specialized region of the nephron
responsible for production and secretion of
the hormone renin, involved in the renin- angiotensin system. This apparatus occurs near the site of contact between the thick
ascending limb and the afferent arteriole.
It contains three components: the macula densa, juxtaglomerular cells, and extraglomerular mesangial cells.[citation needed] Clinical relevance Because of its importance in body fluid
regulation, the nephron is a common target
of drugs that treat high blood pressure and edema. These drugs, called diuretics, inhibit the ability of the nephron to retain
water, thereby increasing the amount of urine produced.
NEPHRON LOOP
In the kidney, the loop of Henle (or Henle's loop or ansa nephroni) is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its discover F. G. J. Henle, the loop of Henle's main function is to create a concentration
gradient in the medulla of the kidney.[citation needed] By means of a countercurrent multiplier system, which utilizes sodium pumps, the
loop of Henle creates an area of high
sodium concentration deep in the medulla,
near the collecting duct. Water present in the filtrate in the collecting duct flows
through aquaporin channels out of the collecting duct, moving passively down its
concentration gradient. This process
reabsorbs water and creates a
concentrated urine for excretion.[citation needed] Structure It can be divided into five parts[citation needed]: Thick descending limb of loop of Henle -- The descending limb has low
permeability to ions and urea, while being highly permeable to water. Thin descending limb of loop of Henle -- The descending limb has low
permeability to ions and urea, while being highly permeable to water. Thin ascending limb of loop of Henle -- The thin ascending limb is not
permeable to water, but it is permeable
to ions. Medullary thick ascending limb of loop of Henle -- Sodium (Na+), potassium (K+) and chloride (Cl-) ions are reabsorbed from the urine by active transport. K+ is passively transported along its concentration gradient through a K+ channel in the apical aspect of the cells,
back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium (Ca2+). Cortical thick ascending limb -- The
cortical thick ascending limb drains
urine into the distal convoluted tubule. The loop has a sharp bend in the renal medulla.[citation needed] Blood supply Counter current multiplier diagram The loop of Henle is supplied by blood in a
series of straight capillaries descending
from the cortical efferent arterioles. These
capillaries (called the vasa recta; recta is from the Latin for "straight") also have a countercurrent multiplier mechanism that prevents washout of solutes from the
medulla, thereby maintaining the
medullary concentration. As water is
osmotically driven from the descending
limb into the interstitium, it readily enters the vasa recta. The low bloodflow through
the vasa recta allows time for osmotic
equilibration, and can be altered by
changing the resistance of the vessels' efferent arterioles.[citation needed] Also, the vasa recta still has the large
proteins and ions which were not filtered
through the glomerulus, which provides an oncotic pressure for ions to enter the vasa recta from the interstitium.[citation needed] The main function of the Loop of Henle is to
set up a concentration gradient.
gradient in the medulla of the kidney.[citation needed] By means of a countercurrent multiplier system, which utilizes sodium pumps, the
loop of Henle creates an area of high
sodium concentration deep in the medulla,
near the collecting duct. Water present in the filtrate in the collecting duct flows
through aquaporin channels out of the collecting duct, moving passively down its
concentration gradient. This process
reabsorbs water and creates a
concentrated urine for excretion.[citation needed] Structure It can be divided into five parts[citation needed]: Thick descending limb of loop of Henle -- The descending limb has low
permeability to ions and urea, while being highly permeable to water. Thin descending limb of loop of Henle -- The descending limb has low
permeability to ions and urea, while being highly permeable to water. Thin ascending limb of loop of Henle -- The thin ascending limb is not
permeable to water, but it is permeable
to ions. Medullary thick ascending limb of loop of Henle -- Sodium (Na+), potassium (K+) and chloride (Cl-) ions are reabsorbed from the urine by active transport. K+ is passively transported along its concentration gradient through a K+ channel in the apical aspect of the cells,
back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium (Ca2+). Cortical thick ascending limb -- The
cortical thick ascending limb drains
urine into the distal convoluted tubule. The loop has a sharp bend in the renal medulla.[citation needed] Blood supply Counter current multiplier diagram The loop of Henle is supplied by blood in a
series of straight capillaries descending
from the cortical efferent arterioles. These
capillaries (called the vasa recta; recta is from the Latin for "straight") also have a countercurrent multiplier mechanism that prevents washout of solutes from the
medulla, thereby maintaining the
medullary concentration. As water is
osmotically driven from the descending
limb into the interstitium, it readily enters the vasa recta. The low bloodflow through
the vasa recta allows time for osmotic
equilibration, and can be altered by
changing the resistance of the vessels' efferent arterioles.[citation needed] Also, the vasa recta still has the large
proteins and ions which were not filtered
through the glomerulus, which provides an oncotic pressure for ions to enter the vasa recta from the interstitium.[citation needed] The main function of the Loop of Henle is to
set up a concentration gradient.
Subscribe to:
Posts (Atom)